Science Is Not Properly Communicated!
Yes, science is really not properly communicated. At present, the method of dissemination is either through an academic conference, a poster, a speaking engagement, or through a research article. Here is an example of an abstract of a research paper taken from Nature Molecular Psychiatry titled "Attention-Deficit Hyperactivity Disorder In Adults: A Systematic Review And Meta-Analysis Of Genetic, Pharmacogenetic, and Biochemical Studies" shown below:
The adult form of attention-deficit/hyperactivity disorder has a prevalence of up to 5% and is the most severe long-term outcome of this common disorder. Family studies in clinical samples as well as twin studies suggest a familial liability and consequently different genes were investigated in association studies. Pharmacotherapy with methylphenidate (MPH) seems to be the first-line treatment of choice in adults with attention-deficit hyperactive disorder (ADHD) and some studies were conducted on the genes influencing the response to this drug. Finally some peripheral biomarkers were identified in ADHD adult patients. We believe this work is the first systematic review and meta-analysis of candidate gene association studies, pharmacogenetic and biochemical (metabolomics) studies performed in adults with ADHD to identify potential genetic, predictive and peripheral markers linked specifically to ADHD in adults. After screening 5129 records, we selected 87 studies of which 61 were available for candidate gene association studies, 5 for pharmacogenetics and 21 for biochemical studies. Of these, 15 genetic, 2 pharmacogenetic and 6 biochemical studies were included in the meta-analyses. We obtained an association between adult ADHD and the gene BAIAP2 (brain-specific angiogenesis inhibitor 1-associated protein 2), even after Bonferroni correction, with any heterogeneity in effect size and no publication bias. If we did not apply the Bonferroni correction, a trend was found for the carriers allele 9R of dopamine transporter SLC6A3 40 bp variable tandem repeat polymorphism (VNTR) and for 6/6 homozygotes of SLC6A3 30 bp VNTR. Negative results were obtained for the 9-6 haplotype, the dopamine receptor DRD4 48 bp VNTR, and the enzyme COMT SNP rs4680. Concerning pharmacogenetic studies, no association was found for the SLC6A3 40 bp and response to MPH with only two studies selected. For the metabolomics studies, no differences between ADHD adults and controls were found for salivary cortisol, whereas lower serum docosahexaenoic acid (DHA) levels were found in ADHD adults. This last association was significant even after Bonferroni correction and in absence of heterogeneity. Other polyunsaturated fatty acids (PUFAs) such as AA (arachidonic acid), EPA (eicosapentaenoic acid) and DyLA (dihomogammalinolenic acid) levels were not different between patients and controls. No publication biases were observed for these markers. Genes linked to dopaminergic, serotoninergic and noradrenergic signaling, metabolism (DBH, TPH1, TPH2, DDC, MAOA, MAOB, BCHE and TH), neurodevelopment (BDNF and others), the SNARE system and other forty genes/proteins related to different pathways were not meta-analyzed due to insufficient data. In conclusion, we found that there were not enough genetic, pharmacogenetic and biochemical studies of ADHD in adults and that more investigations are needed. Moreover we confirmed a significant role of BAIAP2 and DHA in the etiology of ADHD exclusively in adults. Future research should be focused on the replication of these findings and to assess their specificity for ADHD.
What was your take on this abstract? Here is another abstract from the same issue of Nature Molecular Psychiatry titled "Changes For Clozaprine Monitoring In The United States" shown below:
Clozapine is a unique compound that is particularly effective for treatment-resistant schizophrenia (TRS). The use of clozapine is limited, however, due to the 0.8% risk of agranulocytosis,1 which necessitates a strict monitoring of neutrophil counts to detect early neutropenia and prevent progression to agranulocytosis.
First and foremost, I must admit that one of these is an article -- specifically a 'review' while the other is a "news and commentary" -- which means that the formats are quite different. Still, the abstracts are extremely different. Why?
What about if I show you an abstract from a different journal?
How about the journal 'Nature Chemical Biology'?
Here is an abstract from an article titled "Progress And Prospects For Small-Molecule Bacterial Imaging" shown below:
Fluorescence microscopy is an essential tool for the exploration of cell growth, division, transcription and translation in eukaryotes and prokaryotes alike. Despite the rapid development of techniques to study bacteria, the size of these organisms (1–10 μm) and their robust and largely impenetrable cell envelope present major challenges in imaging experiments. Fusion-based strategies, such as attachment of the protein of interest to a fluorescent protein or epitope tag, are by far the most common means for examining protein localization and expression in prokaryotes. While valuable, the use of genetically encoded tags can result in mislocalization or altered activity of the desired protein, does not provide a readout of the catalytic state of enzymes and cannot enable visualization of many other important cellular components, such as peptidoglycan, lipids, nucleic acids or glycans. Here, we highlight the use of biomolecule-specific small-molecule probes for imaging in bacteria.
I think that these four abstracts illustrate the point. Right about now, the reader (you) might be thinking the following regarding the three abstracts above:
What do those abstracts mean?
What science is being done?
Why are the words and sentences so complicated?
Am I right? Were you thinking any of the three questions above. I know that I would be -- especially, if I had very little of a science background to serve as a starting point when reading them.
Science Communication Should Be Simple
In a recent TED talk by Tyler DeWitt titled "Hey Science teachers -- Make It Fun" the problem with communicating science is discussed in a simple and elegant manner. Tyler is a graduate student at the MIT.
Below are two avenues by which a virus can infect a cell. Given that the 'Zika' virus is spreading among the United States population, the stories below are completely relevant to current stories in the popular news press. I paraphrased the speech by Tyler DeWitt and used 'still images' from his TED talk below.
Story #1 goes as follows:
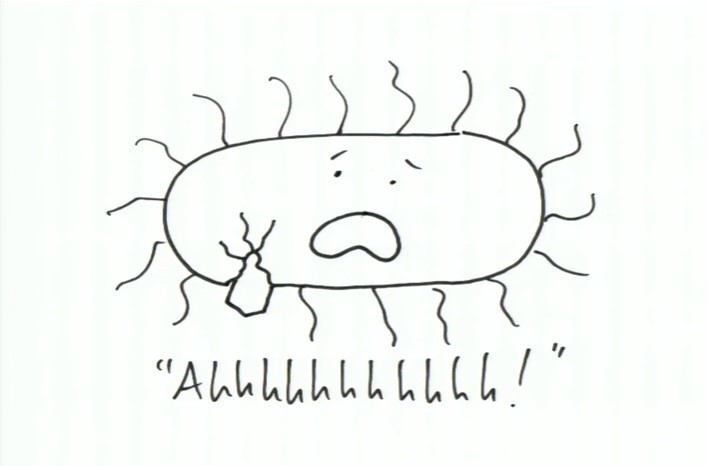
The story starts off with a happy little bacterium who is occupying a medium -- say your stomach. Over time the bacterium starts to not feel well as depicted in the slide below:
While pondering over the many reasons which might lead to a cause for the feeling, he looks down to notice the culprit -- a virus who is emerging from his body as shown below:
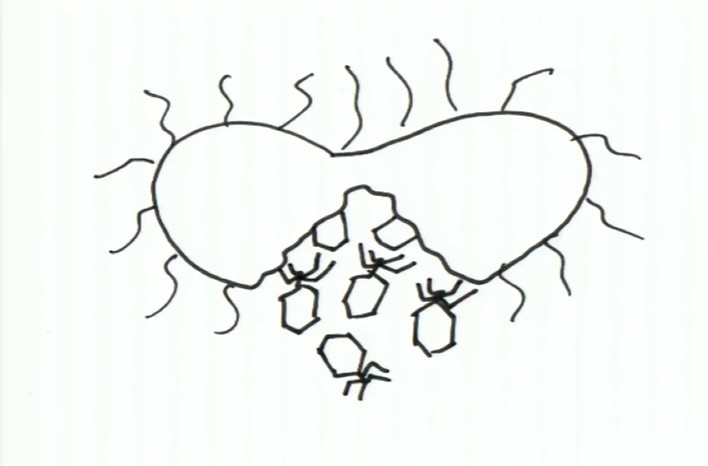
And with time, the situation is getting very worse while the viruses keep poring out from his body as pictured below:
Now there are two different viewpoints to describe the situation that is occurring. From the standpoint of the bacterium, the situation is worsening exponentially with time hosting an army of viruses. While, from the viewpoint of the virus, each little virus is thinking the following:
"We rock!" From the viruses viewpoint, the first virus managed to get into the host and successfully propagate -- evolve. In order to complete the mission of evolving a number of complex steps had to occur for survival to happen. Lets review them from the standpoint of the virus.
First, the virus had to slip a copy of its DNA into the bacterium as shown below:
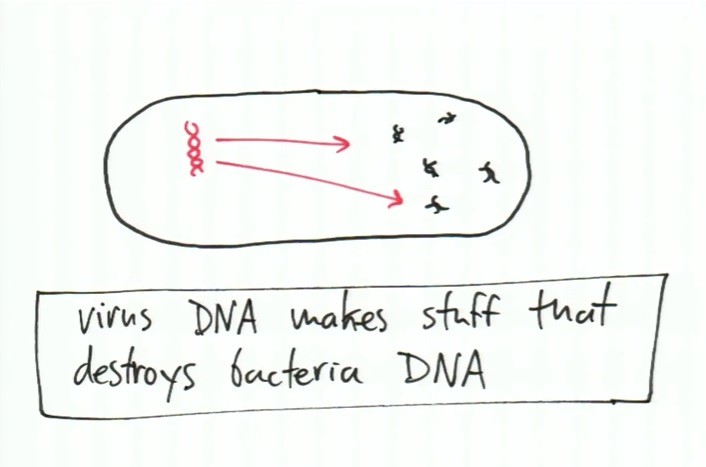
In order for the virus to proceed to copy its DNA, the virus had to destroy the DNA of the bacterium as shown below:
After gaining control of the bacterium by destroying the bacterial DNA, the bacterium will not propagate (copy) only the DNA of the virus. The bacterium is serving as the host factory producing multiple copies of the virus as shown below:
The bacterium will continue to make copies of the virus since the 'blue print' has been changed from the bacterium's DNA to the viruses DNA. Manufacturing the virus will not stop until the bacterium bursts due to holding 'too many copies' of the virus as shown below:
The above steps illustrate one avenue by which viruses "infect" bacteria to takeover as a host.
Is there an alternate way for the virus to invade the bacteria and take over to use as a host?
Yes! There is -- which is outlined below:
The virus starts out as a "secret agent" as shown below:
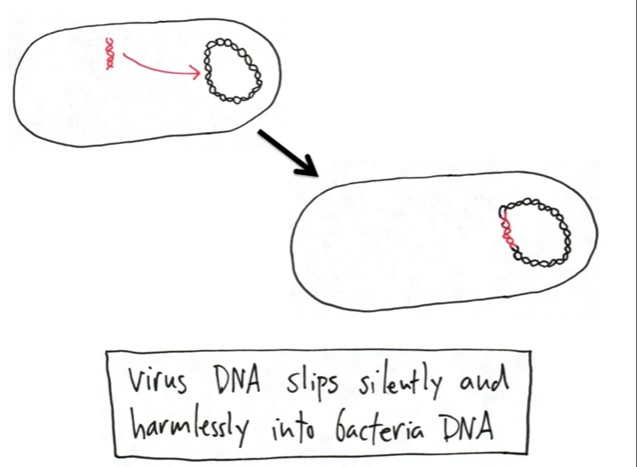
With the ability to secretly insert its DNA into the DNA of the bacterium as shown. The insertion process has no damaging effect like the first avenue of replication did in the example above. The DNA appears to be normal inside the bacterium as shown below:
As mentioned, the secret agent is able to insert his DNA into the bacterium who is unaware of the insertion and lives life normally. Over time, the bacterium reproduces/replicates itself and makes many copies of the "inserted DNA" which has been silent as shown below:
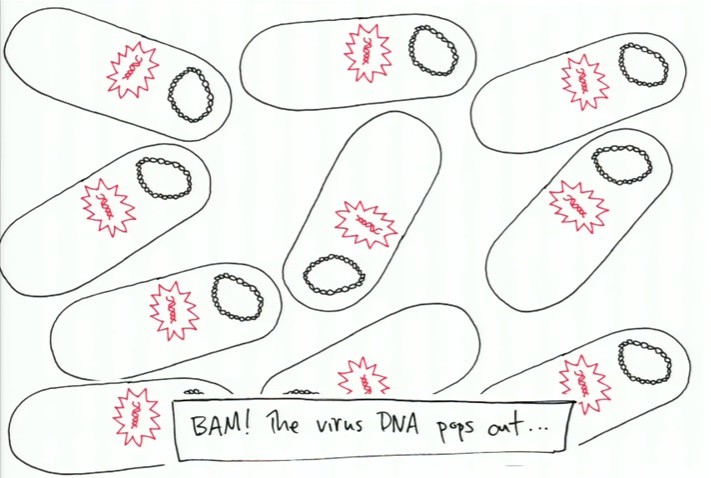
The silent/inactive inserted DNA is not recognizable to the bacterium until a "signal" is sent among the bacterium and the virus DNA pieces pop out and take control over all of the bacterium -- also shown above. After the virus DNA has taken over the bacterium, the replication process of the virus occurs as shown below:
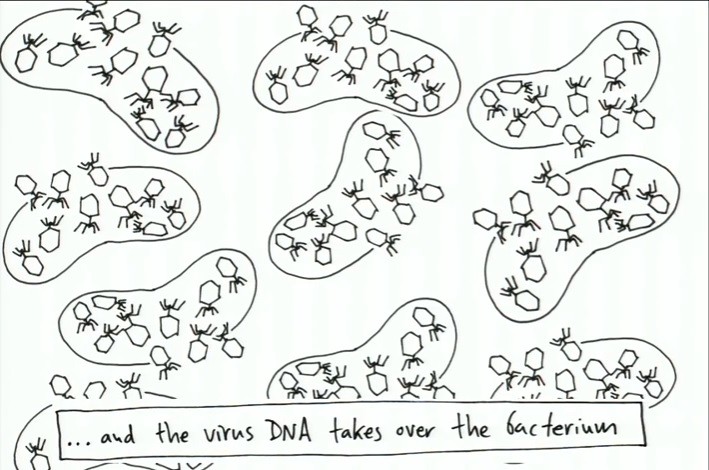
The bacterium have been turned into virus making factories. Extended copies of the virus are produced in each bacterium until the bacterium bursts and releases all of the copies of the viruses as shown below:
And with that, the viruses have won by dominating and replicating through the bacterium. Shown in the slides (which are still pictures taken from Tyler Dewitt's TED talk) are two different stories.
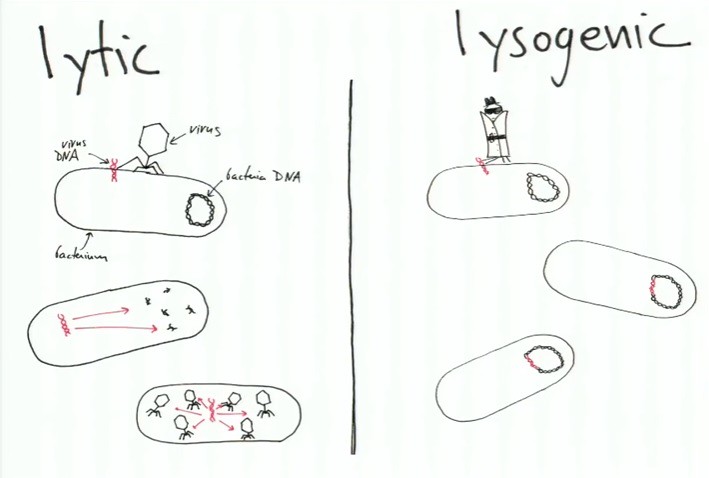
These two stories represent the two pathways by which a virus can attack cells!!!!
On the left hand-side of the picture above is the first pathway (the lytic pathway) -- where the viruses insert themselves and take control over the cell (bacterium) immediately. Whereas, in the second pathway (lysogenic pathway), the virus inserts their DNA and that DNA stays dormant until a signal is sent.
Was that hard to explain and comprehend or what?
All science should be that simple - right?
Virtually, everyone who has graduated high school has been exposed to these two pathways in their biology class. The difference is in the presentation of the material. First, the presentation that each of us experienced was most likely more serious than the cartoon story above and certainly did not use cartoon characters like those presented above.
Why not?
The field of science suffers from a "seriousness" problem. Which is to say, scientists, and the way that science is portrayed is too serious. Science is meant to be fun too. You can have fun doing science. I do it every day!
In the next section before concluding, I will tie together the first two sections. Namely, the seriousness of science -- which is a downfall and -- secondly, the language that is used. Language seems to be the number one 'turn off' for students entering various fields of science.
Science Should Be Simplified!
Many of scientists that I know believe that making science simple is simply impossible. Furthermore, the belief is centered around the idea that "dumbing down" science devalues the field. This belief could not be further from the truth. Let me explain why with more slides from Tyler DeWitt's TED talk above to illustrate my point. There is no need to recreate the wheel.
In Tyler's TED talk, the two stories about the two possible avenues by which a virus can infect a cell were told. And he used cartoons and very creative imagery along with simple words right. Everything he said was easily digestible -- at least for me.
Textbooks often complicate explanations of science as do professional publications (i.e. journal articles -- as shown above). Why do these publications use such complicated language to illustrate a point? Because, that is the way the system is designed to be -- which needs to change.
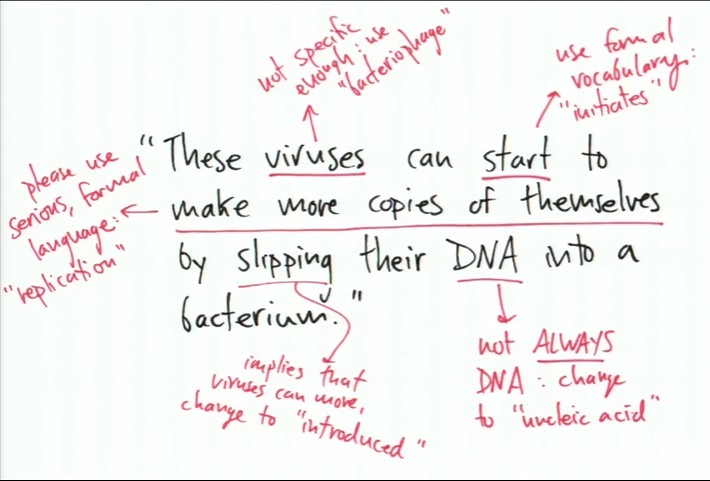
In the example given in the TED talk above, the simple explanation might be something like: Viruses make copies of themselves by slipping their DNA into a bacterium. How would this look in the formal language inside of a textbook? Here is an example -- a slide from the TED talk above with the informal explanation above and the formal explanation below:
Wow. The two descriptions above look completely different. The first (above) is one that I can relate to and would love to read. Whereas the second (below) is completely a 'turn off' and might very well put me to sleep. Here is the divergence of the majority of people's attention. When the practitioners of science transition from the top to the bottom description -- a large percentage of the audience drops off too.
Why does this transition occur in descriptions?
Because, in the simple description, not every word is accurate. After going through and editing the statement for accuracy -- 100% accuracy, the statement would look like the one shown below with corrections:
And this begs the question of science: can we describe science with slightly inaccurate descriptions? I would argue that the answer is yes. Why? Because, a majority of the undergraduate education uses "toy examples" to illustrate the concepts and theories. For example, in the undergraduate curriculum students learn about "ideal gases" and the ideal gas law. The assumption is that gas molecules are "point particles" and do not "interact." What does this mean?
Throughout the undergraduate degree process -- at least in Chemistry -- students are running calculations using the "ideal gas equation" to arrive at relations between chemical compounds. Real gases do not behave ideally and computational coefficients are added to equations to take into account the 'non-linear' behavior. The non-linear behavior arises when gas molecules react with one another or during the collision -- the molecules temporarily "stick together." These types of properties are extremely complex and cannot be simulated with the limited (and great) computational power that society possesses today. See? This is why simplification can work.
Conclusion...Science Should Be Made Simple!
In order to capture the interests of the widest audience for science, the work has to be made simple. A few professors worry about the simplification process attracting others to science. Who cares if that happens? Would we not want the best minds tackling the problems of society? Yes, we would. Science is meant to be fun -- not just serious without.
With captivating and creative descriptions by enthusiastic scientists like Tyler DeWitt, we have a great opportunity to engage a wider audience into science. Although, if other scientists don't sign onto this line of thinking, the new avenue will run dry and we will be stuck with the same old 'broken' method of communicating science.
The world has changed over the decades. Why shouldn't the communication evolve too to attract the widest array of audience members? Technology is providing a whole new range of possibilities for the classroom to teach the message of science. Why would we want to stick with the same broken method that has accomplished enough for the past few decades -- but is currently in need of an overhaul.
One of the overarching goals of this blog is to simplify science for everyone. If you have an idea that you would like explained on the blog site, please leave a comment. As you can see by reading the first few abstracts (descriptions of science studies in section one), we have a long way to go. Lets all band together and demand a change of our system to move toward a more creative and captivating educational system for all disciplines. Until next time, Have a great day.