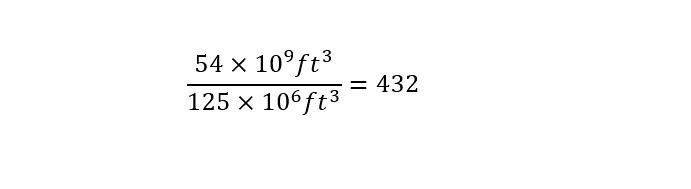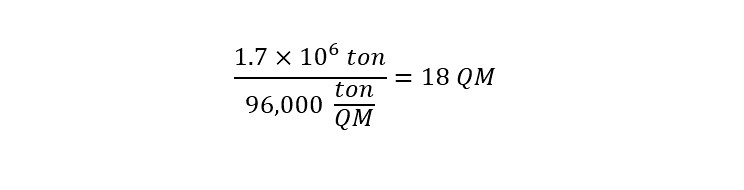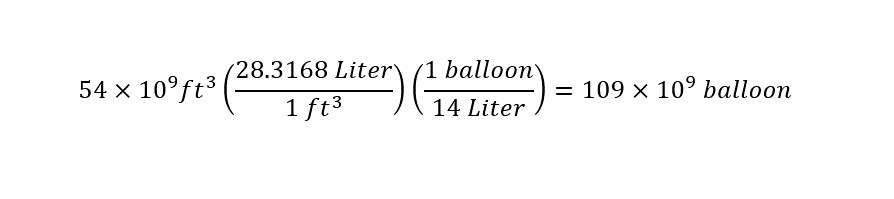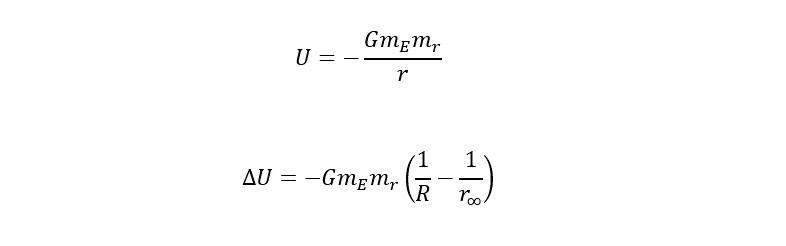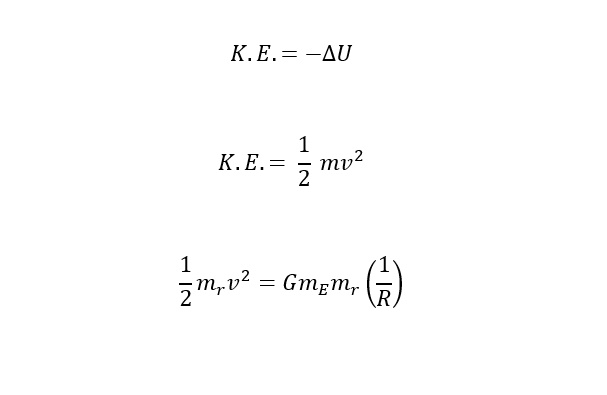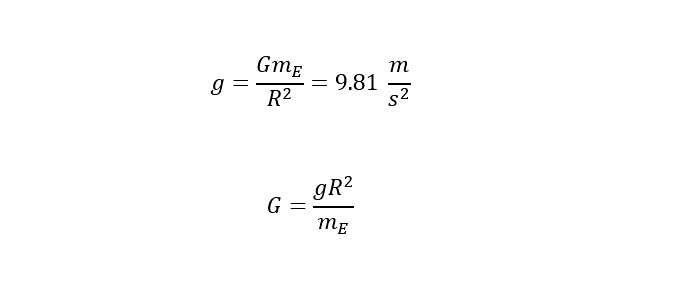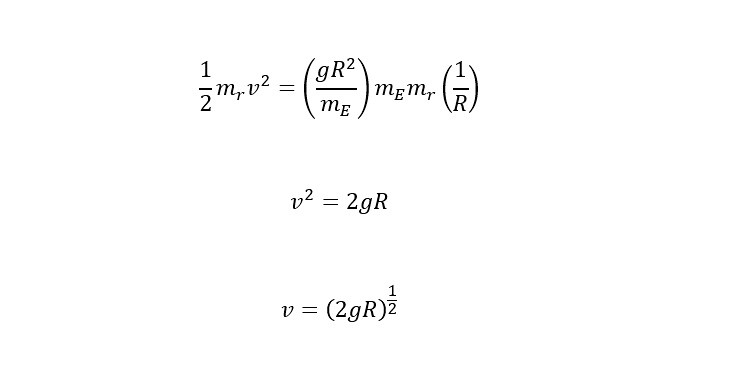Recently, a large helium deposit was found off of Tanzania in Africa that holds an estimated 54 billion cubic feet. WOW! Thinking of helium from the perspective of providing "lift" in a balloon, how much weight could the amount contained in the reserve (in Africa) lift? Read on to find out the answer.
How Much Volume Does 54 Billion Cubic Feet Of Helium Occupy?
As readers of this blog site know, one of the many goals is to demystify science along with the large numbers and concepts that come with the field. Recently, an article titled "Scientists Make Huge Helium Discovery (On Planet Earth)" appeared on the website "ChemInfo" that unveiled a new helium deposit that has been found in Africa. Here is an excerpt detailing the new discovery and the importance from the article:
The scientific community has been abuzz for years that the earth is running out of helium. Though it is one of the most abundant resources in the universe, helium is in limited supply on earth. And once it’s extracted and used, it’s gone forever.Helium is a rare and vital commodity with many uses including industrial leak detection and deep sea diving equipment. Liquid helium is also used as a coolant for rocket fuel and as a superconductor for magnets in MRI machines. Plus, kids love it at parties.Because our stockpile of helium has been dwindling at the National Helium Reserve in Texas, with no known new helium gas fields to extract it from, concerns have mounted that the days of floaty party balloons could soon be ending. Recently, scientists made a major discovery that could change that.For the first time, researchers have pinpointed a giant helium gas field. Usually, helium is discovered accidentally when extracting oil and gas. Up until this point, scientists had never intentionally located a helium field. But the discovery was made after a team from the University of Oxford and Durham University collaborated with a Norwegian helium exploration company, Helium One, and set out to see if it could be done.
If you are wondering whether the world is getting desperate for helium, you are correct. As the world consumes helium through the avenues listed above, including the semiconducting industry (the largest user), reserves are becoming more precious.
This is why researchers are becoming "proactive" in their search as described above. Just think about the prospect of not being able to get an MRI at the hospital after sustaining an injury -- due to the lack of a magnetic field which is dependent upon metal windings being immersed in a bath of liquid helium. Once charged, the metal windings hold the current circulating at zero resistance (superconductivity) until the wires become room temperature again (helium boils off).
How much helium was discovered?
Here is another excerpt regarding the discovery amount shown below:
The researchers estimate that the field could be holding as much as 54 billion cubic feet of helium, which is more than twice the amount being currently held in the U.S. If correct, the field could help supply the world’s helium for generations to come.Assuming the detection methods are accurate, the team says the next step will be finding the best place to extract the gas. They also hope that the discovery could potentially lead to scientists uncovering more helium fields.
Sticking with the past blog posts on this site, how does a person comprehend such a volume of helium? First, I should point out that the amount is in question due to the measurement device. That is an important bit of information. Why? The amount detected is only as accurate as the measurement device. Furthermore, just because the amount is assumed to be that large, the actually amount extracted could be different.
How different?
Probably no more than an order of magnitude.
In order to visualize the enormous amount of helium that has recently been discovered in Africa, lets return to an old post. As you will recall, there was an enormous amount of gas leaked from a storage facility in Southern California -- the Aliso Canyon Gas Storage Facility -- starting the end of last year. The leak was massive enough that the total amount of gas leaked turned out to be around 5 billion cubic feet leaked.
The underground storage reservoir was estimated to hold around 184 cubic feet of gas. A reader sent in a comment to correct me and said that the gas was under extreme pressure, therefore, the total space of the reservoir was less (but how much less) -- not too much less. The fact remains that the amount of helium discovered in Africa is within a range of volume that has been put into perspective.
Specifically, how many Mercedez Benz Super Domes would have to be filled to hold 54 billion cubic feet of helium gas?
In order to carry out the calculation, the volume of the Super Dome needs to be known -- which is around 125 million cubic feet interior volume. A picture of the exterior of the Super Dome is shown below:
Source: Photo by David Grunfeld (Nola.com)
With the interior volume of the Super Dome known to be 125 million cubic feet, the amount of Super Domes that could be filled is calculated by dividing the two numbers as shown below:
Wow! The amount of gas that was discovered underground is enough to fill 432 Mercedez Benz SuperDomes? My goodness, that is a large amount of gas. I am constantly amazed at the volumes that are listed in these news stories. Like most readers, I read the same statistic -- 54 billion cubic feet -- of helium and think the following: That must be an enormous amount?
By performing dimensional analysis, I am one step closer by comparing the amount to other statistics in the news, such as the Porter Ranch Gas Leak I mentioned above. Dimensional analysis allows me to place the number in a better place -- a place in my mind of perspective. Store that number next to Porter Ranch under the mental file -- excessive amounts of gas -- really incomprehensible.
The question that was intended to be answered in the blog post was the following:
How much weight can 54 billion cubic feet of gas lift?
This requires us to think like blimp designers. Lets do some math below to find out.
How Much Weight Can 54 Billion Cubic Feet Of Gas Lift?
To tackle the question of the blog post, we must think like a blimp designer to a small extent. In theory, a blimp is filled with helium and can provide a large amount of lift to accomplish a task. The blimp in question could look like the following -- only much larger:
Source: ABC News
Shown above is the 'Goodyear' Blimp. According to the website 'HowStuffWorks.com,' the average blimp holds a volume of gas in the range of 67,000 -- 250,000 cubic feet of helium. Since the volume of gas that is contained in the ground in Africa is enormous -- nearly 5 orders of magnitude larger, our imagination will have to suffice. Just think of the blimp above just many orders of magnitude larger -- use creativity.
In order to calculate the amount of weight that 54 billion cubic feet of gas can lift, a ratio of volume to weight needs to be determined for a balloon. I found the answer on 'StuffYouShouldKnow.com' in a post titled "How Many Balloons Would It Take to Lift You Off The Ground, Answered" for the amount of balloons required to lift a person off of the ground. Lets start by learning from 'frames' taken in the video to determine how many balloons are required to lift a person off of the ground. From there, the volume can be changed to the volume in question and then determine the weight!
Here are the slides with the relevant information to answer the following question:
How many balloons does it take to lift a person off of the ground?
First, the amount of helium needs to be known for a single balloon along with the weight that can be lifted as shown below:
Next, the volume in a typical balloon needs to be known as shown below:
Next, the logic amounts to the following:
1) If 1 Liter of helium can lift 1 gram, then 14 Liters of helium will lift 14 grams.
2) If a person weighs 50 Kg = 110 pounds, then 3500 balloons should lift them off of the ground?
The last statement is shown with no calculations -- which is bothersome if you are the author of this blog site -- right? In light of this stretch, I calculated the amount of balloons needed to lift 50 Kg as shown below using the video producers logic:
Therefore, 50,000 grams of weight will require 50,000 Liters of helium. If each balloon holds 14 Liters, then dividing 50,000 Liters by 14 Liters will yield the number of balloons. In this case, the number calculated revealed just over 3500 balloons would be needed. The number coincided with the number stated in the video above.
Therefore, I felt comfortable with the ability to reproduce the number reported in the video with the values (parameter values) listed in the video. This is important for reproducing results. Reproducing results is increasingly important in science today more than ever with the exponentially growing number of studies being published hourly.
Next, using the values in the video -- I calculated the weight (number of tons).
Wow. As expected, that is a large amount of weight that can be lifted by 54 billion cubic feet of helium. How can that number be put into perspective? In a previous post, the weights of two large objects (Airplane: Spruce Goose and Ship: Queen Mary) were compared and contrasted.
How about using the weight of the Queen Mary -- which was 96,000-tons?
How many Queen Mary Ships can 54 billion cubic feet of helium lift?
Before the calculation are shown, a picture of the Queen Mary ship might assist you in putting the enormous weight into perspective. Here is a picture of the Queen Mary below taken from 'Wikipedia':
Source: By RMS_Queen_Mary_Long_Beach_January_2011 -- David Jones
The calculation is shown below:
According to the calculation, 54 billion cubic feet of helium contained in a 'Goodyear Blimp' could lift the equivalent weight to 18 Queen Mary ships. WOW. That is a large amount of helium. I guess now I understand the enormous enthusiasm behind the discovery off of the tip of Africa.
Just for fun, I thought that I would calculate the number of balloons that could be filled (for celebrations: birthday parties, anniversaries, graduations, etc.). I show the calculation of the number of balloons that could be filled with 54 billion cubic feet of helium below:
That is an incomprehensible number -- 109 billion balloons. That would be an enormous number of celebrations. More than during the span of our lifetime.
Have I made the point yet?
What point?
Conclusion
The discovery of an underground reserve of helium off of Africa has the potential to supply the world with helium for a few decades. This is reassuring, especially, since just a couple of years ago, the government started discussing the possibility of "rationing" helium to the world. The current discover is a relief to say the least. How big is the discovery? I hope that I have put the enormous number into perspective for you in the paragraphs above.
In a future post, I will explain further why the discovery is important to the field of science. I now have a few more useful statistics to compare large numbers too -- and so do you. The next time that someone asks you how to visualize 54 billion cubic feet of a gas (such as helium), you will be able to quickly answer them with the handy statistics:
1) The equivalent of 432 Mercedez Benz SuperDomes!!!
2) If the volume was contained in a blimp, the blimp could lift 18 Queen Mary ships!!!
3) If the volume was in party balloons, 109 billion balloons could be filled for celebrations!!!
Until next time, have a great day!