In the following paragraphs, I will explain how chemistry followed me into the military. Specifically, I will highlight two separate environments -- high school and the military to illustrate my point -- your passion/interests are constantly intersecting your life. Do you believe me? If not, read more below. If so, read more below.
When Did Chemistry Appeal To Me?
Growing up, my father would always talk to me about chemistry. Part of that is due to that he loved chemistry. He is a true academic in the sense that he could get lost in studying science. If he were to be taken hostage and locked up in a library, given the proper amount of food and clothing, he would live the remainder of his life happy as ever. I remember when I was in Junior High, he put a bumper sticker on his car that read "Honk If You Got an A In P-Chem." Who would have thought that two decades later I would become a "physical chemist."
My first exposure in academia to chemistry was kind of "off the beaten path." I used to "ditch" classes quite a bit. I missed a lot of high school one particular semester. As a result, I was given a punishment. First, I would attend Saturday detention from 8 am - 12 pm. I remember my father proudly dropping me off to attend. He was happy that I received a proper punishment for missing school. Additionally, I had to skip lunch and report to the chemistry/physics teacher's classroom -- Mr. Barth -- now Dr. Barth.
What seemed like a punishment then, turned into a major part of my doctoral work a decade later. I was given the task of building (with a friend) a track of alternating bar magnets. The track was to be two magnets wide (around 4 inches) and around 6 feet long. In total, there were around 250 magnets that we had to glue (opposite polarity) alternating (north to south). At this point, you might ask the following question:
What was the purpose of the experiment?
In short, the object was to build a "magnetic levitation train" to measure the coefficient of friction. Before I answer the question in detail, a visual diagram of the experimental setup would be very useful in interpreting the purpose of the experiment. The experimental setup when completed appeared like the following photograph of the "kit" that sells today online:
Source: www.rainbowresource.com
In the diagram above, there appears to be a block of wood that is floating. On either side of the track, there are plastic rails to hold the block of wood or magnetic car onto the track. Back in the late 80s, our car was simply made out of cardboard with magnets glued onto the bottom. There is a fair amount (a huge) of tedious work involved in building the track. That process too prepared me for research in the physical science area.
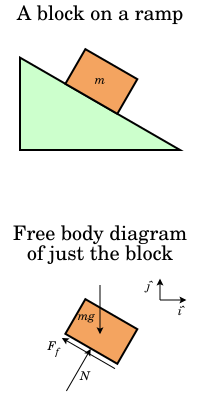
The purpose of the track was to elevate one side of the track to form a "triangle." The diagram would appear to be similar in nature to a block of wood sliding down a slanted surface. Additionally, if the relevant forces are outlined, the diagram taken from the "Wikipedia" page emerges:
Source: By Krishnavedala
By studying the above diagram, the forces are outlined. In the past, "force" has been introduced in another blog post as the product of the mass of an object with the gravitational acceleration constant pushing the object toward Earth. Therefore, the only new concept is the force of friction. Friction is created all around us. Stop reading this blog and rub your hands together. Do they start to heat up? That is due to the friction between the two surfaces of your hands. Got it! Good.
With a magnetic levitating track, where is the friction? The only source of friction (neglecting wind resistance) is due to the car (cardboard) rubbing up against the plastic rails on the track. By changing the angle of the track relative the the ground and measuring the time of travel, the coefficient of friction is easily determined. That was our challenge.
I say "our" because there was another gentlemen in the room assigned to the project. He did not miss school like me. In fact, he was a straight "A" student. He had a name -- Gil Vitug. We became and remain very good friends. At the time, he was more attracted to the physics side of life. Years later, we both graduated with our doctorate degrees (Ph.D.) from University of California at Riverside. He was working in Astrophysics (working at the Stanford Linear Accelerator) while I was working on developing instrumentation for Nuclear Magnetic Resonance experiments.
From that experience, both of us learned the ability to extract a large amount of information from a low-cost setup. Finding a way with limited funding to measure a quantity is extremely useful. Especially, as science funding is becoming more difficult to receive. That was a valuable experience and served as a springboard to which we became "science ambassadors." Out of our school class, we were the two to work in academia.
After high school, I entered college and majored in chemistry with the intention of becoming a surgeon. I wanted to end up in experimental medicine. I even defined my own field -- experimental medicine. Today, that desire would have translated to obtaining a "Md/Ph.D" degree and working in a government laboratory. I had no clue at the time. In fact, my father sat me down and had a talk with me during my junior year of college. He suggested that I look into graduate school in chemistry rather than medicine based on my responses to his questions regarding experimental medicine. I was at the time and remain extremely grateful for that discussion.
Why did I diverge onto that tangent?
Out of those experiences, came a love for chemistry. The experiences were not traditional to me. Late night discussions with my father over topics such as dropping a penny into a bottle of beer spurred my interests in thinking about chemistry. I was not a good student in school. I did show up every day to class. And, I was able to entertain concepts in science reasonably well. The concepts would be in my head.
What remained to be a delinquency was the patience to sit down and study along with explaining the concepts contained within my head. The process of beginning to tackle that delinquency took up the better part of the next decade. Although, with the help of certain individuals (like my father and Dr. Bath along with Gil -- now Dr. Vitug) and a military sergeant, the path was easier. Each person challenges me to become a better person. Furthermore, optimizing the shortcomings in my life has been a continuous challenge -- still to this day. Let me explain briefly how.
Chemistry In The Military?
How can a soldier study chemistry in the military? As I mentioned in a previous blog post, chemistry is all around us. Everything involves chemistry! What determines whether a soldier studies or utilizes chemistry is their job classification or rank. If an enlisted soldier decides to become an officer, he/she returns to college and majors in science. That could involve returning to a job in the military that involves directly performing research.
Although, the more probable situation would be to assigned a job where the requirements have no direct connection to chemistry. Additionally, as an enlisted soldier, the job is most likely going to entail no direct connection to research in sciences. That is reserved more for a position like an officer or a civilian employee.
I was assigned to work as an electrician on the fighter aircraft F-16. That entailed working on the jet on the "flight line" along with working on the parts in a "back shop" setting. What is the difference between the two: "flight line" and "back shop"? Working on the "flight line" involves removing electrical components (generators, rheostats, controllers, batteries, chargers, etc.) and environmental components (bleed air valves, air condition controllers, water separation units, etc.) along with repairing the associated wiring and ducting to those components.
This is different from working in the "back shop" or the component repair shop. The component repair shop is a The two types of work are very different but have the same mission. The overall mission is to keep aircraft in the air. With that being said, work that arrives in the "back shop" or component repair shop can be from any aircraft -- not just the F-16. Since our base (Shaw AFB, South Carolina) was a predominantly F-16 air base, most of the components that we encountered to repair were from F-16 aircraft.
What does all this have to do with chemistry and being a chemistry ambassador?
When I first arrived at the base, my supervisor -- Master Sergeant Daniel Jonas asked me a series of questions. These included if I had any college or university experience. I answered yes -- I had 4 years in chemistry before dropping out. He scolded me for dropping out and encouraged me to finish my degree in the military (and become an officer). He also sent me to the "Middle East" 18 months out of the 24 months -- due to my popularity (hard work ethics). Even though I did not get to go back to school while serving my country, I had the ability to demonstrate my knowledge of the field of chemistry by an assignment -- which was an interesting and unusual occurrence in the military. Especially for an enlisted soldier in his/her first tour of duty.
Master Sergeant Daniel Jonas was a curious man. In fact, he had an unquenchable thirst for information -- spanning all disciplines from economics through physical sciences. He was a very interesting person to say the least. I have often wondered how I happen to run across people in my life like him -- I am extremely fortunate. My wife says, I attract these people -- who see my potential. Maybe she is correct.
Anyways, Msgt. Jonas realized an issue with a battery and called on my chemistry skills to fix the problem. Specifically, he was concerned about two aspects of recharging (or reconditioning) the F-16 battery. First, the unusually large amount of waste generated in the process of charging the battery. Second, the methodology of charging the battery which degraded the lifetime of the battery -- which was nominally around 3-5 years. Let me explain the situation using science language.
Hazardous Waste Generation
The F-16 battery is a single unit (one case) that houses 24 cells that are linked together in "series." A picture of the battery is shown below:
Source: Public Domain
With the diagram of each "cell" shown below:
Source: By Ransu, Public Domain
In order to understand the problems that Msgt. Jonas recognized, the chemical reactions of the discharging and charging cycle of the battery need to be known. Shown below are the chemical reactions of the two cycles of the Nickel Cadmium battery taken from the patent webpage for the "battery charger":
Upon inspection of the chemical reactions, the hydroxide ions play a critical role in the discharge/charge cycle over the course of the life of the battery. The electrolyte solution must contain a chemical that upon dissociation produces a hydroxide ion. For the battery above, the chemical is a solution of potassium hydroxide in water. This is important in recognizing the problem that needed to be fixed to extend out the life of the battery.
I was tasked to understand the charging/discharging cycle of the battery. Furthermore, I was tasked with explaining the problem to the other members of the back shop working on the batteries. Before I go into that, the charging cycle needs to be understood. Looking at the "Wikipedia" page for the "Nickel-Cadmium Battery" the process proceeds like in the following manner:
Vented cell (wet cell, flooded cell) NiCd batteries are used when large capacities and high discharge rates are required. Traditional NiCd batteries are of the sealed type, which means that charge gas is normally recombined and they release no gas unless severely overcharged or a fault develops. Unlike typical NiCd cells, which are sealed, vented cells have a vent or low pressure release valve that releases any generated oxygen and hydrogen gases when overcharged or discharged rapidly. Since the battery is not a pressure vessel, it is safer, weighs less, and has a simpler and more economical structure. This also means the battery is not normally damaged by excessive rates of overcharge, discharge or even negative charge.They are used in aviation, rail and mass transit, backup power for telecoms, engine starting for backup turbines etc. Using vented cell NiCd batteries results in reduction in size, weight and maintenance requirements over other types of batteries. Vented cell NiCd batteries have long lives (up to 20 years or more, depending on type) and operate at extreme temperatures (from −40 to 70 °C).A steel battery box contains the cells connected in series to gain the desired voltage (1.2 V per cell nominal). Cells are usually made of a light and durable polyamide (nylon), with multiple nickel-cadmium plates welded together for each electrode inside. A separator or liner made of silicone rubber acts as an insulator and a gas barrier between the electrodes. Cells are flooded with an electrolyte of 30% aqueous solution of potassium hydroxide (KOH). The specific gravity of the electrolyte does not indicate if the battery is discharged or fully charged but changes mainly with evaporation of water. The top of the cell contains a space for excess electrolyte and a pressure release vent. Large nickel plated copper studs and thick interconnecting links assure minimum effective series resistance for the battery.The venting of gases means that the battery is either being discharged at a high rate or recharged at a higher than nominal rate. This also means the electrolyte lost during venting must be periodically replaced through routine maintenance. Depending on the charge–discharge cycles and type of battery this can mean a maintenance period of anything from a few months to a year.Vented cell voltage rises rapidly at the end of charge allowing for very simple charger circuitry to be used. Typically a battery is constant current charged at 1 CA rate until all the cells have reached at least 1.55 V. Another charge cycle follows at 0.1 CA rate, again until all cells have reached 1.55 V. The charge is finished with an equalizing or top-up charge, typically for not less than 4 hours at 0.1 CA rate. The purpose of the over-charge is to expel as much (if not all) of the gases collected on the electrodes, hydrogen on the negative and oxygen on the positive, and some of these gases recombine to form water which in turn will raise the electrolyte level to its highest level after which it is safe to adjust the electrolyte levels. During the over-charge or top-up charge, the cell voltages will go beyond 1.6 V and then slowly start to drop. No cell should rise above 1.71 V (dry cell) or drop below 1.55 V (gas barrier broken).
The take home point was that there was maintenance involved in the discharging/charging process over the course of the life of the battery. My supervisor wondered why the life of the battery was no where near the length that was written by the factory. This is where my job started -- since I had a chemistry background and interest in science.
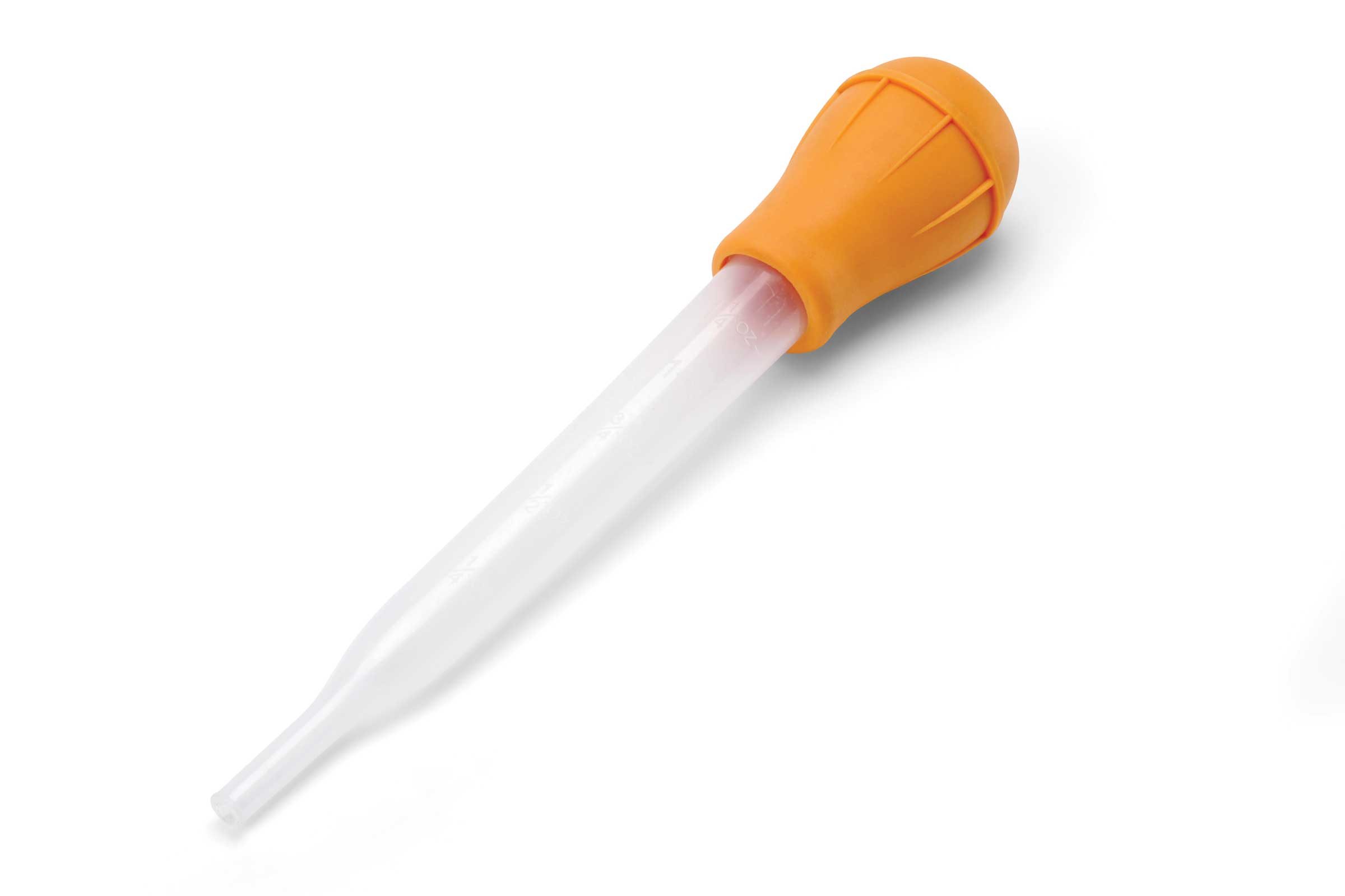
To accommodate the expansion of the volume of liquid during the charging cycle, each instrument had a "turkey baster" sitting next to it for the easy removal of excess water. During the dynamic charging cycle, the cells would expand due to the hydrogen gas being liberated. The caps would be loosened and set beside the battery. Essentially, the battery sat on the table top hooked up the charger and "open" (vent caps removed) to the environment. Unknown to us at the time, that is where the problems lay the entire time -- the open cells to the atmosphere. Why?
Source: www.rd.com
There were a couple of issues with the charging/disharging cycles that I started to mention above which may be confusing. After the charging cycle, the "electrolyte" level might need to be adjusted (meaning removal or addition of water with the "turkey baster" device shown above) as discussed in the excerpt above.
The problem with this is the removal of the following: 1) electrolyte mixture -- KOH and H20 (Potassium hydroxide and water), and 2) the electrode (which decomposed). Collecting these two chemicals is and disposing them safely (not down the drain) is required. This means that the solution of waste has to be kept in a "hazardous waste" container -- which is picked up each week by a disposal company. Each weak, the shop would generate on the order of 55 gallons of "hazardous waste" -- mostly water, but a little bit of potassium hydroxide, electrode (cadmium, nickel, etc.). As you might imagine, this was a huge motivation to determine how to extend the life of the battery.
During the addition of water or the extraction of the electrolyte after charging, the problem was that the internal concentrations of all components had changed. If the "turkey baster" was used to pull out water/KOH and electrode material, the over the course of the lifecycle of the battery -- each time that the battery was sent to be conditioned in the "back shop" -- the battery would be degraded ever so slightly. Adding this up over time, renders the battery unusable.
Couple this to the competing chemical reaction occurring with the air -- which is shown below:
This reaction was not known to occur at the time of our investigation. If Msgt. Jonas had not been so persistent in understanding all chemical reactions within the F-16 battery, the situation (short lifetime of the battery) would have continued on for decades. What did I learn out of this? Does any of this make sense to you (the reader)? I know that I have been rambling on for a while.
Conclusion....
The point I would like to make with this post is that a persons true passion becomes apparent eventually in one's life -- whether they pursue work within that passion or not. For Master Sergeant Jonas, that passion is an unquenchable thirst for knowledge. He is a power house of knowledge and commands those around him "in directly" to be thirsty as well. Amazing. I have always loved chemistry in one form or another. Dr. Dan Barth has taught chemistry and physics for decades. My father shares a passion for the physical sciences (as well as others too). Put all of us in a room together or have us interact with each other, and these shared interests will become apparent soon. Additionally, each one will show their specific talent or interests over time.
Regardless if a person pursues their interests or not, those interests will become apparent over time. For me, hanging out in the chemistry and physics classroom benefitted me greatly -- since this experience was aligned with my interests. I imagine that the school counselor who assigned me to the room instead of detention saw my interests shine through at some point in our interactions.
Similarly, when I arrived in the US Air Force at Shaw AFB -- I must have exuded the interests in sciences. This later caused me to be chosen to interpret and explain the work of Master Sergeant Jonas and the extension of the F-16 battery. What does this have to do with you?
If you are at a point in your life where you have no idea of where to go in moving forward, just keep moving forward. Eventually, your interests will come to the surface. But, you must be willing to listen to yourself and observe your interests. I will you luck in your adventure pursuing your interests. Have a great day.



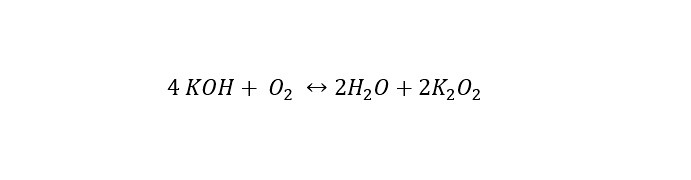
No comments:
Post a Comment