As modern medicine moves toward the field popularized by the term "personalized medicine," along the way, the first stop will be gender specific medicine. At least, that seems likely as the field of medicine continues to evolve and research unveils gender specific treatments and disease types.
Gender Specific Diseases?
Each of us have encountered different statistics in the healthcare field for diseases based on gender. One such example is the occurrence of cardiovascular disease in adults over the age of 40 years. For women, the occurrence is 1 in every 2 whereas for men it is 2 in every 3. The obvious question arises:
Why do these differences arise?
Recently, I was listening to a TED talk titled "
His and Hers ... Healthcare" by Dr. Paula Johnson. The video runs less than 15 minutes in length and is worth watching.
In her talk, she starts off with citing statistics in occurrence of mental illness between men and women. Which ultimately leads her to the visionary statement of the differences lie at the molecular level leading to "sex differences in each cell". This is visionary way of thinking. Before I tell you more about the TED talk by Dr. Paula Johnson and the differences in treatment for men and women in medicine, lets look at Dr. Paula Johnson's TED talk profile.
Here is an excerpt from the page:
Dr. Johnson is the Executive Director of the Connors Center for Women's Health and Gender Biology, Chief of the Division of Women's Health at Brigham and Women's Hospital in Boston, Massachusetts and a Professor of Medicine at Harvard Medical School. As an entrepreneurial leader in medicine, she has built organizations which stand at the leading edge of hospital-based interdisciplinary healthcare delivery, discovery and disease prevention. Dr. Johnson started and grew the Connors Center for Women's Health and Gender Biology. This nationally-recognized center, includes an interdisciplinary health care practice model that solidifies the important connection between healthcare delivered to each patient and the health of entire communities.
Read more by clicking the hyperlinked sentence introducing the excerpt above which came from the personal profile composed by the TED talk organization. I just wanted to give the reader some background on the evolution of Dr. Paula Johnson. Dr. Johnson introduces through a brief account of the emergence of the National Institutes of Health's (NIH) 'Revitalization Act of 1993' -- which was critical to the inclusion of minorities and women into clinical trials.
Here is a summary amended in 2001 from the NIH website:
SUMMARY: This notice updates the NIH policy on the inclusion of women and minorities as subjects in clinical research. It supercedes the 1994 Federal Register notice (https://grants.nih.gov/grants/guide/notice-files/not94-100.html) and the August 2000 notice in the NIH Guide to Grants and Contracts (https://grants.nih.gov/grants/guide/notice-files/NOT-OD-00-048.html). It incorporates the definition of clinical research as reported in the 1997 Report of the NIH Director’s Panel on Clinical research. Also, this notice provides additional guidance on reporting analyses of sex/gender and racial/ethnic differences in intervention effects for NIH-defined Phase III clinical trials. The guidelines ensure that all NIH-funded clinical research will be carried out in a manner sufficient to elicit information about individuals of both sexes/genders and diverse racial and ethnic groups and, particularly in NIH-defined Phase III clinical trials, to examine differential effects on such groups. Since a primary aim of research is to provide scientific evidence leading to a change in health policy or standard of care, it is imperative to determine whether the intervention or therapy being studied affects women or men or members of minority groups and their subpopulations differently.
In June 2001, NIH adopted the definition of clinical research as: (1) Patient-oriented research. Research conducted with human subjects (or on material of human origin such as tissues, specimens and cognitive phenomena) for which an investigator (or colleague) directly interacts with human subjects. Excluded from this definition are in vitro studies that utilize human tissues that cannot be linked to a living individual. Patient-oriented research includes: (a) mechanisms of human disease, (b) therapeutic interventions, (c) clinical trials, and (d) development of new technologies; (2) Epidemiologic and behavioral studies; and (3) Outcomes research and health services research http://www.nih.gov/news/crp/97report/execsum.htm.
Imagine that before the inclusion of minorities and women, all of medical trial data was based on men?
Is that really possible?
Upon the first read, this might be impossible to believe.
Even drug testing?
Yes, this is possible. I know from direct experience. Back in 1995, I was taking a polymer chemistry course from an old chemist named Professor Roy Kreiger. Professor Kreiger had been in industry as a bench chemist before he returned to academia to teach. During our lecture he told of a weekend which was spent as a bench chemist having an extreme case of feeling nauseous. Here is the story.
He was working as a bench chemist at a pharmaceutical company. Over the weekend (during time off), the company offered its employees an opportunity to earn "extra money". Sometimes he would participate and during other weekends he would not. The participation would entail taking the experimental medicine over the weekend and returning to work on Monday to fill out a detailed questionnaire regarding the experience. He did this up until he had a bad reaction.
After vomiting all weekend, he returned to work to report that the voluntary medication caused him to be nauseous all weekend. When he asked what the experimental medication was that he had been ingesting all weekend, the response was that he was administered a new type of "birth control."
After that experience, he no longer participated in earning "extra money". Upon seeing the TED talk by Dr. Paula Johnson, I was reminded that there was a time when drugs were only tested on men. That time was when Professor Kreiger worked in the pharmaceutical industry.
Returning to the video above, Dr. Paula Johnson provides two distinct examples of physiological differences between men and women in disease. Below the picture is a description of the differences of the two images by Dr. Paula Johnson taken from her TED talk above.
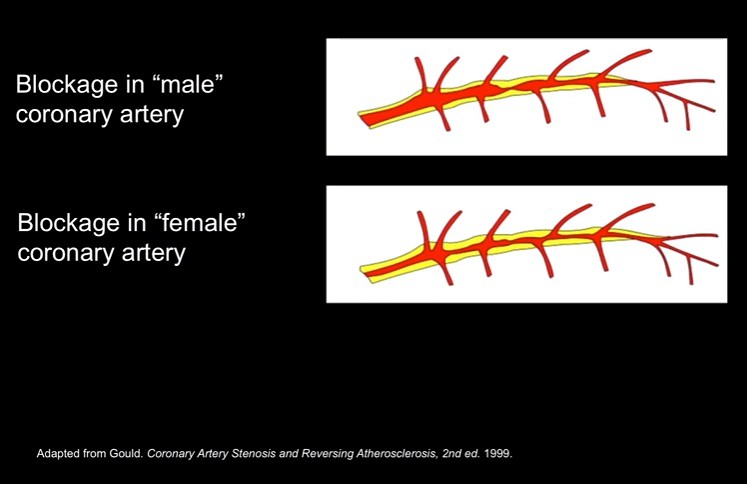
The first example is the difference in plaque build up in the artery as shown below:
Here is the description of the difference by Dr. Paula Johnson of the slide above:
Let's start with heart disease. It's the number one killer of women in the United States today. This is the face of heart disease. Linda is a middle-aged woman, who had a stent placed in one of the arteries going to her heart. When she had recurring symptoms she went back to her doctor. Her doctor did the gold standard test: a cardiac catheterization. It showed no blockages. Linda's symptoms continued. She had to stop working. And that's when she found us. When Linda came to us, we did another cardiac catheterization and this time, we found clues. But we needed another test to make the diagnosis. So we did a test called an intracoronary ultrasound, where you use soundwaves to look at the artery from the inside out.
And what we found was that Linda's disease didn't look like the typical male disease. The typical male disease looks like this. There's a discrete blockage or stenosis. Linda's disease, like the disease of so many women, looks like this. The plaque is laid down more evenly, more diffusely along the artery, and it's harder to see. So for Linda, and for so many women, the gold standard test wasn't gold.
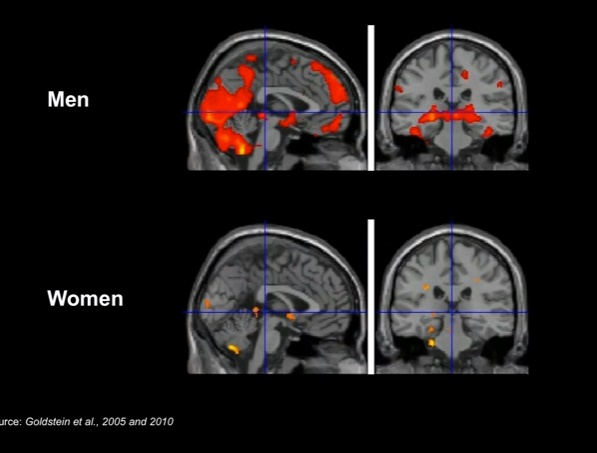
and the second example is a functional MRI (Magnetic Resonance Imaging) image of depression in both men and women. The brain areas highlighted in distinct regions as shown below:
Here is the description of the slide by Dr. Paula Johnson in her TED talk above:
So let's go back to depression. Depression is the number one cause of disability in women in the world today. Our investigators have found that there are differences in the brains of women and men in the areas that are connected with mood. And when you put men and women in a functional MRI scanner -- that's the kind of scanner that shows how the brain is functioning when it's activated -- so you put them in the scanner and you expose them to stress. You can actually see the difference. And it's findings like this that we believe hold some of the clues for why we see these very significant sex differences in depression.
But even though we know that these differences occur, 66 percent of the brain research that begins in animals is done in either male animals or animals in whom the sex is not identified.
So, I think we have to ask again the question: Why leave women's health to chance? And this is a question that haunts those of us in science and medicine who believe that we are on the verge of being able to dramatically improve the health of women. We know that every cell has a sex. We know that these differences are often overlooked. And therefore we know that women are not getting the full benefit of modern science and medicine today. We have the tools but we lack the collective will and momentum.
There are other factors that could contribute to differences in the above images. Although, overall, there are major difference as shown above. These two examples are among many that highlight the need to have different clinical trials and treatment for different sexes. Dr. Paula Johnson makes a compelling case to change the system based on the examples that she uses in her TED talk. She also points out that the system has yet to change completely -- which is surprising to me. If differences were apparent, why would we as a society not want to treat everyone equal -- find a treatment for each of us. This is a bridge toward providing "personalized medicine".
In the paragraphs below, I show an example of private funding, published research, and new research arising which are addressing the differences in sexes with regard to research and development.
In order to change the system, the challenge will take time. Although, as you will see, progress is being made which suggests motivating evidence of a change. If you are interested in reading on and looking at the various abstracts of journal articles and books, please feel free to do so. Additionally, a few foundations and their work are shown below which cast light on the much needed research due to differences in gender.
Today, research with the inclusion of sex and minorities is nowhere where it needs to be -- in terms of taking into account the sex differences of disease.
Why? Is the cost to expensive? Do some researchers feel like they are duplicating a trial? Who knows.
Supplemental Material below:
Organizations Concerned About Gender Medicine
Dr. Paula Johnson highlights in her talk that despite the obvious data from various studies (some of which) introduced in her talk, 60 % of studies are still using either men or rats as test subjects. Why? Obvious, the inclusion of women and minorities is still at its infancy. More discussion and relevancy needs to be brought to the forefront of the decision making process.
How do we accomplish this as a society?
The process could begin with funding projects from private funding. Part of the money which comes from private funding sources may originate from a foundation. Below are a couple of foundations which are built to elevate the importance of gender or sex in carrying out research clinical trials.
1)
Foundation for Gender-Specific Medicine:
OUR MISSION
To use the study of gender to foster the development of new sciences and improve health care for all patients
The Foundation for Gender Specific-Medicine supports the investigation of the ways in which biological sex and gender affect normal human function and the experience of disease. One of the discipline’s pioneers, Marianne J. Legato, FACP, MD established the Foundation as a continuation of her work with The Partnership for Gender-Specific Medicine at Columbia University.
OUR GOALS
1. Support original scientific research in gender-specific medicine:
Each year, the Foundation provides fellowships to untenured, young faculty members with the goal of fostering their interest in gender-specific medicine at the beginning of their investigative careers. Currently, we award two-year research grants at the Columbia University College of Physicians and Surgeons and one-year grants at the Johns Hopkins School of Medicine. However, we are always looking for new scholars.
2. Create an evidence-based set of protocols to guide physicians:
The Foundation is working to assemble a critical mass of evidence-based criteria for optimal gender-specific treatment within each specialty of medicine. We have finished recommendations for gender-specific care of diabetics and are currently working on cardiovascular disease.
Gender-specific care centers around the globe will receive our completed guidelines. We will then observe and summarize the impact of these guidelines based on the morbidity and mortality of patients treated according to our recommended protocols. Columbia University’s Office of Clinical Trials will collaborate with us in organizing and supervising our clinical studies on the impact of sex and gender on the efficacy of new drugs and medical devices.
3. Educate of the lay public and the scientific/medical community:
The Public
The Foundation understands that science does not operate outside of the rest of society, and we consider education a central part of our mission. The interests of the lay public drive medical research and practice. Rather than simply serving as an informational vehicle, the Foundation creates an open dialogue between patients and the medical community. In addition to the many books that Dr. Legato has written for the lay public, the Foundation promotes gender-specific medicine through lectures, symposia, and social media.
The Foundation for Gender-Specific Medicine is heavily invested in elevating awareness of the differences arising from sex or gender in diseases and treatments. Columbia University is not a 'unknown' university and stands at the forefront of research in medicine. Also listed on the page "about us" is the range of organizations that the foundation is affiliated with. Many are home to Nobel Prize winners. More and more people are interested in finding out the answer of how much contribution gender or sex plays into the role of disease or treatment.
2)
FONDAZIONEISTUD:
Below is an abstract for a review of Gender Medicine titled: "
Gender Medicine: A new approach for healthcare"
Abstract:
Gender Medicine is a fascinating newly emergent approach of medicine aimed at recognizing and analyzing the differences arising from gender in several aspects: anatomical, physiological, biological, functional, social and in the field of the response to pharmacological treatment. The term gender is to be intended as the definition issued by the World Health Organization (WHO), according to which gender refers to the socially constructed roles, behaviors, activities, and attributes that a given society considers appropriate for men and women. Therefore, Gender Medicine deals with a wider area than simply taking into account sex differences, which are merely the biological characteristics that define men and women.
Several studies have demonstrated that the physiology and the psychology of men and women are different and this diversity has a profound impact on the development, diagnosis and treatment of a disease and also on how the patient deals with that pathology. Gender Medicine applies these concepts in order to ensure everyone the best available treatment, with several benefits: it reduces the level of error in medical practice, promotes therapeutic appropriateness for both genders and improves personalized therapies, finally lowering the costs of the National Health Services (NHS), in a long term perspective.
The aim of this project is to drive awareness of this emergent topic. We firstly defined Gender Medicine, by analyzing the contents and by a historical overview, from its first steps to concrete applications, both in Italy and in the international context, thanks to interviews with national and international experts. We then moved to the analysis of clinical features: when, how and why the outcomes of drug therapies are different according to gender? We also described the perceptions of the involved stakeholders (physicians, patients, institutions, etc.) and we finally asked people for their opinion on the topic, through an online questionnaire.
Foundations and projects which emerge from them are crucial to the emergence and mainstream inclusion of women and minorities into clinical and research trials. Although, until different studies start to show up in the scientific journals as "published articles" -- the idea will remain at a distance. Even when the evidence is present. Below are a few published reviews or papers highlighting the need for inclusion of sex differences in to research that have emerged in the last few years:
1)
Handbook of Experimental Pharmacology:
"
Sex and gender differences in clinical medicine"
Abstract:
Sex and gender differences in frequent diseases are more widespread than one may assume. In addition, they have significant yet frequently underestimated consequences on the daily practice of medicine, on outcomes and effects of therapies. Gender medicine is a novel medical discipline that takes into account the effects of sex and gender on the health of women and men. The major goal is to improve health and health care for both, for women as well as for men. We give in this chapter an overview on sex and gender differences in a number of clinical areas, in cardiovascular diseases, pulmonary diseases, gastroenterology and hepatology, in nephrology, autoimmune diseases, endocrinology, hematology, neurology. We discuss the preferential use of male animals in drug development, the underrepresentation of women in early and cardiovascular clinical trials, sex and gender differences in pharmacology, in pharmacokinetics and pharmacodynamics, in management and drug use. Most guidelines do not include even well-known sex and gender differences. European guidelines for the management of cardiovascular diseases in pregnancy have only recently been published. Personalized medicine cannot replace gender-based medicine. Large databases reveal that gender remains an independent risk factor after ethnicity, age, comorbidities, and scored risk factors have been taken into account. Some genetic variants carry a different risk in women and men. The sociocultural dimension of gender integrating lifestyle, environment, stress, and other variables cannot be replaced by a sum of biological parameters. Because of this prominent role of gender, clinical care algorithms must include gender-based assessment.
The wide range of diseases where sex differences is prominent are so large that one would think that the field of 'Gender Medicine' would explode. Catchy titles like the following were used to get the attention of professionals in order to get traction.
2)
Clinical Chemistry Laboratory Medicine:
Research Article: "
Gender medicine: a task for the third millennium"
Abstract:
Gender-specific medicine is the study of how diseases differ between men and women in terms of prevention, clinical signs, therapeutic approach, prognosis, psychological and social impact. It is a neglected dimension of medicine. In this review we like to point out some major issues in five enormous fields of medicine: cardiovascular diseases (CVDs), pharmacology, oncology, liver diseases and osteoporosis. CVDs have been studied in the last decades mainly in men, but they are the first cause of mortality and disability in women. Risk factors for CVD have different impacts in men and women; clinical manifestations of CVD and the influence of drugs on CVD have lot of gender differences. Sex-related differences in pharmacokinetics and pharmacodynamics are also emerging. These differences have obvious relevance to the efficacy and side effect profiles of various medications in the two sexes. This evidence should be considered for drug development as well as before starting any therapy. Gender disparity in cancer incidence, aggressiveness and prognosis has been observed for a variety of cancers and, even if partially known, is underestimated in clinical practice for the treatment of the major types of cancer. It is necessary to systematize and encode all the known data for each type of tumor on gender differences, to identify where this variable has to be considered for the purposes of the prognosis, the choice of treatment and possible toxicity. Clinical data suggest that men and women exhibit differences regarding the epidemiology and the progression of certain liver diseases, i.e., autoimmune conditions, genetic hemochromatosis, non-alcoholic steatohepatitis and chronic hepatitis C. Numerous hypotheses have been formulated to justify this sex imbalance including sex hormones, reproductive and genetic factors. Nevertheless, none of these hypothesis has thus far gathered enough convincing evidence and in most cases the evidence is conflicting. Osteoporosis is an important public health problem both in women and men. On the whole, far more epidemiologic, diagnostic and therapeutic studies have been carried out in women than in men. In clinical practice, if this disease remains underestimated in women, patients' and physicians' awareness is even lower for male osteoporosis, for which diagnostic and therapeutic strategies are at present less defined. In conclusion this review emphasizes the urgency of basic science and clinical research to increase our understanding of the gender differences of diseases.
3)
World Journal of Gastroenterology:
Research Article: "
Gender specific medicine in liver diseases: a point of view"
Abstract:
Gender medicine focuses on the patho-physiological, clinical, prevention and treatment differences in diseases that are equally represented in men and women. The purpose of gender medicine is to ensure that each individual man and woman receives the best treatment possible based on scientific evidence. The concept of "gender" includes not only the sexual characteristics of individuals but also physiological and psychological attributes of men and women, including risk factors, protective/aggravating effects of sexual hormones and variances linked to genetics and corporal structures that explain biological and physiological differences between men and women. It is very important to consider all the biological, physiological, functional, psychological, social and cultural characteristics to provide patients with individualized disease management. Herein, we critically analyze the literature regarding gender differences for diseases and acquired conditions of the most representative hepatic pathologies: primary biliary cirrhosis, autoimmune hepatitis, primary sclerosing cholangitis, non alcoholic fatty liver disease and alcoholic liver disease, and viral chronic hepatitis B and C. The last section addresses hemochromatosis, which is a prevalent iron overload disorder in the Caucasian population. This review aims to describe data from the literature concerning viral chronic hepatitis during pregnancy, management during pregnancy and delivery, and new effective drugs for the prevention of maternal infection transmission without significant adverse effects or complications.
4) Journal
Atherosclerosis:
Research Article: "
Sex differences in cardiovascular risk factors and disease prevention"
Abstract:
Cardiovascular disease (CVD) has been seen as a men's disease for decades, however it is more common in women than in men. It is generally assumed in medicine that the effects of the major risk factors (RF) on CVD outcomes are the same in women as in men. Recent evidence has emerged that recognizes new, potentially independent, CVD RF exclusive to women. In particular, common disorders of pregnancy, such as gestational hypertension and diabetes, as well as frequently occurring endocrine disorders in women of reproductive age (e.g. polycystic ovary syndrome (PCOS) and early menopause) are associated with accelerated development of CVD and impaired CVD-free survival. With the recent availability of prospective studies comprising men and women, the equivalency of major RF prevalence and effects on CVD between men and women can be examined. Furthermore, female-specific RFs might be identified enabling early detection of apparently healthy women with a high lifetime risk of CVD. Therefore, we examined the available literature regarding the prevalence and effects of the traditional major RFs for CVD in men and women. This included large prospective cohort studies, cross-sectional studies and registries, as randomised trials are lacking. Furthermore, a literature search was performed to examine the impact of female-specific RFs on the traditional RFs and the occurrence of CVD. We found that the effects of elevated blood pressure, overweight and obesity, and elevated cholesterol on CVD outcomes are largely similar between women and men, however prolonged smoking is significantly more hazardous for women than for men. With respect to female-specific RF only associations (and no absolute risk data) could be found between preeclampsia, gestational diabetes and menopause onset with the occurrence of CVD. This review shows that CVD is the main cause of death in men and women, however the prevalence is higher in women. Determination of the CV risk profile should take into account that there are differences in impact of major CV RF leading to a worse outcome in women. Lifestyle interventions and awareness in women needs more consideration. Furthermore, there is accumulating evidence that female-specific RF are of influence on the impact of major RF and on the onset of CVD. Attention for female specific RF may enable early detection and intervention in apparently healthy women. Studies are needed on how to implement the added RF's in current risk assessment and management strategies to maximize benefit and cost-effectiveness specific in women.
The research has been covered for at least the last 9 years, but remains invisible to the public. Specific journals cover the field (as is the case in other specialized areas of science). Although, with the importance arising between sex differences, one would think that the research would be broadcasted to a wider audience.
Journal that is discontinued after running for 9 years is "
Gender Medicine" with the last issue highlighting the transition toward the use of genomics in medicine shown below:
1)
Publication: Gender Medicine
Research Article: "
Mainstreaming Sex and Gender Analysis in Public Health Genomics"
Abstract:
The integration of genome-based knowledge into public health or public health genomics (PHG) aims to contribute to disease prevention, health promotion, and risk reduction associated with genetic disease susceptibility. Men and women differ, for instance, in susceptibilities for heart disease, obesity, or depression due to biologic (sex) and sociocultural (gender) factors and their interaction. Genome-based knowledge is rapidly increasing, but sex and gender issues are often not explored.
2)
Handbook of Clinical Gender Medicine :
Book Description:
A new vision to understanding medicine
Gender medicine is an important new field in health and disease. It is derived from top-quality research and encompasses the biological and social determinants that underlie the susceptibility to disease and its consequences. In the future, consideration of the role of gender will undoubtedly become an integral feature of all research and clinical care.
Defining the role of gender in medicine requires a broad perspective on biology and diverse skills in biomedical and social sciences. When these scientific disciplines come together, a revolution in medical care is in the making. Covering twelve different areas of medicine, the practical and useful Handbook of Clinical Gender Medicine provides up-to-date information on the role of gender in the clinical presentation, diagnosis, and management of a wide range of common diseases.
The contributing authors of this handbook are all experts who, in well-referenced chapters, cogently and concisely explain how incorporation of gender issues into research can affect the medical understanding and treatment of heart disease, osteoporosis, arthritis, pain, violence, and malaria among other conditions. This intriguing and unique medical textbook provides readers with a valuable new perspective to understand biology and incorporate gender issues into the different branches of medicine.
And last but not least, an emerging field (little late after 20 years) is the
International Society of Gender Medicine whose
mission is stated below:
Aims of the IGM
The specific purpose of the society is to establish and develop gender medicine in an international context by promoting gender –specific research in basic sciences, clinical medicine and public health. This is based on the insight, that the two sexes may have different experiences of the same disease: they may present with different symptoms, respond differently to therapy and tolerate/cope with the disease differently. The pathophysiology of disease may also vary as a function of genetics, epidemiology and biological sex/gender.
Therefore, the society will aim to:
1) Advance the understanding of sex/gender differences by bringing together scientists and clinicians of diverse backgrounds;
2) Strive to implement gender in the medical curriculum, prepare and allocate gender-specific learning materials, curricula and gender trainings for instructors
3) Promote gender-specific public health issues such as information for persons, institutions and organizations in the area of gender medicine
4) Facilitate interdisciplinary research on sex/gender differences in basic and clinical frameworks
5) Encourage the application of new knowledge of sex/gender differences to improve health and health care
6) Cooperate with other professional and international societies of gender medicine and similar scientific organizations
7) Encourage and support the creation of professional organizations dedicated to the promotion of sex/gender medicine GM
8) Encourage and support international cooperation, collaboration and education among professionals working in the field sex/gender medicine.
9) Organize international meetings and congresses on relevant topics
10) Assist in the publication of position papers and guidelines in credible scientific journals and textbooks
There is a strong need for a society like the International Society of Gender Medicine to exist. Healthcare professionals should gather together to explore the emerging field. As mentioned earlier, the field of Gender Medicine is relatively new. The most probable cause for the infancy of the field is the lack of 'gender specific care' or stigma associated with a 'non-binary' gender classification.
As highlighted in the abstracts of the emerging articles, research and development is making apparent the need to include minorities and women into trials and clinical research design. The path toward "personalized medicine" involves dealing with the wide spectrum of gender types that compose our society today. Using the pre-historic 'binary system' (male or female) classification is no longer valid. More important is to understand the gender differences that actually arise in our society today and have been around for quite sometime -- just not recognized.
Until next time, Have a great day!