Source: Indiana University
How does the medical community collect medical knowledge? Through research efforts at University hospitals? Through research efforts at private medical facilities? Do these different entities share information freely among one another? The answer to the last question is "no" unfortunately. Although, groundbreaking results are shared through publishing in medical journals which disseminate the knowledge to the world at large. What am I getting at?
There is a lack of 'collective information' on the present state of 'medicine' -- which might sound rather strange at first pass to the reader (you). If a person stops to think about how information is gathered to advance science (in general) then the problem might just present itself in a better light with regard to the lack of dissemination of knowledge within the medical community. Even reading that there is a lack of knowledge in the medical community might raise alarm in readers minds -- which is good -- considering the proposed change by the National Institutes of Health. First, lets look at drug discovery briefly.
How are drugs discovered?
As I wrote about in a previous post, the process of drug discovery begins at the university stage. Basic research begins at the university with funding provided by the National Science Foundation along with the National Institutes of Health -- two large government funding agencies. Of course, there are a whole host of other possible funding sources, but the top two are undoubtedly the NSF and the NIH. During this research and discovery period, scientists are looking for basic targets that are associated with diseases or other biological functions.
If a discovery is made, then that target -- site on a protein or other biological surface might be passed onto the biotech or chemical industry to develop drugs (pharmaceutical) or chemicals (pesticides -- chemical industry) -- to name a couple of routes. The take home message is that research done at this stage is purely discovery and not necessarily money driven. Unlike the research which is conducted in industry settings which must yield profits or be discontinued.
The cost of such research in industry is large. Why? Because, as I stated in a previous blog -- the total cost of bringing a drug to market (i.e. pharmacy shelf) is around 20 billion dollars -- yes, "$20,000,000,000.00"-- which is very expensive. The development of a drug target into an actual treatment is costly from a research standpoint. Not to mention, the legal paperwork, the marketing, and other regulatory paperwork that is involved in the process. Even with assistance from government funding agencies like the NIH and the NSF, the cost of bringing a drug to the pharmacy counter is costly.
As a result of this cost, information (data) regarding the drug trials (clinical trials) is secretive and held by the drug manufacturer. Even though the drug was ultimately produced with money from the tax-payers, the information is proprietary. Most drug companies tend to keep such information very close and secretive. This results in the information on patient variability or patient participation that is kept secret and not for government or public researchers to access. Such information would be extremely beneficial to public researchers and should be made public for mining or searching.
Overall, patient information in research settings and medical practice settings are kept secret from the world. The results/information is very important and should be available since the information shows the differences in patient variability. Differences in treatment and differences in outcomes. What if that information was made available to incorporate into public research? How much better off would the medical community be?
Personalized Medicine
There is a tremendous amount of variation that is present throughout the human population. Each of us have similarities but many differences too. The practice of medicine has relied on the sharing of information throughout the world. A great deal of that sharing has been either through participation at conferences or through the dissemination of knowledge/results in medical/science journals which publish research results. Additionally, a small amount of advances have been carried by the media. The media could do a large amount more but unfortunately, research results do not always attract viewer attention compared to salacious scandals involving politicians and corporations.
What if we paid more attention to the results of medicine?
How can the public get involved in the effort to do so?
Especially, given the lack of understanding in participation?
After all, patients hear about clinical trials which are underway through their respective physicians or by learning about the possibility of participation via the internet. Of course, that takes a tremendous amount of effort (not really) on each human part. The main obstacle toward achieving greater solutions in medicine is sharing medical files.
At present, each of us go to the doctor to be examined and treated for an ailment (problem). A medical file (medical folder) is created at the doctor's office with the medical history and the medical issue along with the recommended treatment (as prescribed by the physician) him/herself. That medical information is not 'shared' with researchers around the world. Which is a large issue. Why? The motivation for change can be stated as the following:
Imagine all of the medical files that are kept at all of the different doctors offices around the world which are not being shared. In order to share them, each patient needs to sign a 'release form' to whichever physician or agency he/she would like to share their medical history with. This means that there is a tremendous (huge, enormous) amount of medical history about the citizens of the world that is kept 'private' and not accessible to the research community. That information could be extremely useful to understand the different types (of variability) of diseases which exist in the human population. Until that medical history is shared, then medicine will rely on the few cases which are shared (which is very little compared to the human population).
What if the ability to share each of our personal medical information was possible? What if the government had access to a million medical files? Imagine the amount of information that could be obtained? The opportunity just arose with the unveiling of the initiative called "All of Us" by the National Institutes of Health. Here is the mission statement (brief) on the webpage linked:
The All of Us Research Program is a historic effort to gather data from one million or more people living in the United States to accelerate research and improve health. By taking into account individual differences in lifestyle, environment, and biology, researchers will uncover paths toward delivering precision medicine.
Below is a video which serves to motivate the reason why the "All Of Us" project is important:
See? A testimonial video is shown below of the personal impact that 'personalized medicine' has had on a silicon valley investor - Eric Dishman:
Here is another description from Indian University shown below:
Imagine if researchers had the enormous amount of medical files that are reported to be possible by the "All of Us" initiative by the National Institutes of Health? All of the patient information which displays the variability (differences) between 1 million patients. Plus, the similarities in disease type and treatment would be known too. Which would help researchers understand to a greater extent what works and what does not for a given disease (or type of disease). Everyone wins when we understand the differences between various citizens walking the Earth.
Conclusion...
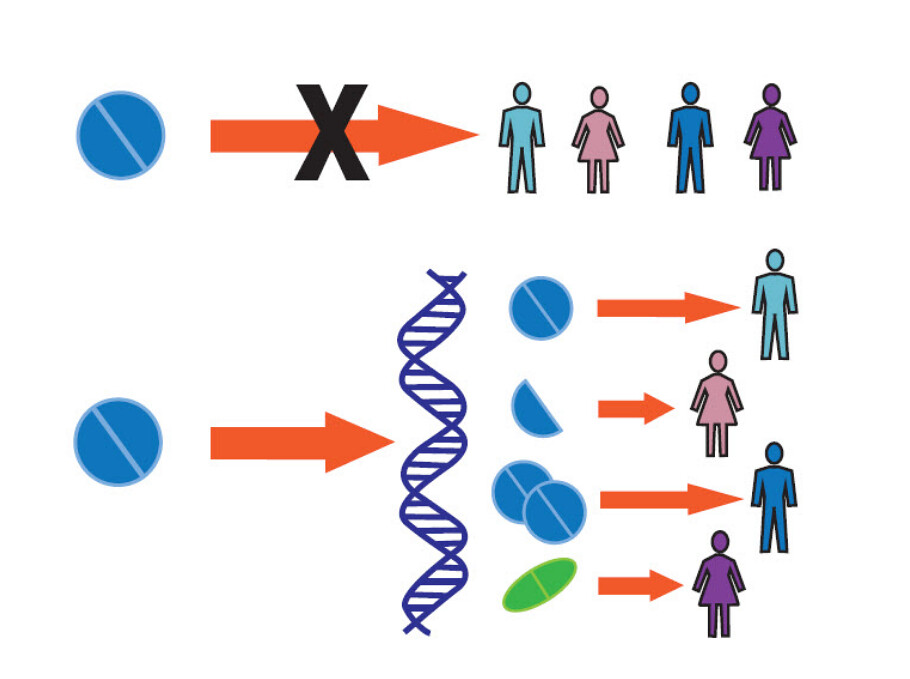
The photo at the beginning of the blog post shows two horizontal lines indicating two different paths of treatment. In the first path (the top) is a single pill which is given to four different patients. There is a large red 'X' indicating that precision medicine does not aim to continue to give 'one treatment' to everyone and expect a 'good outcome.' Whereas in the second (lower) horizontal treatment path, the same 'pill' is shown to be blocked en route to the patient by a strand of DNA. Further, the original path is split into four different treatment paths for four different patients. This indicates that the treatment which was previously thought to treat four different people is insufficient. Upon accessing greater knowledge regarding variability in the patient population, the one treatment path turns into four different treatment paths for four different people. As indicated a patient might get the same pill, whereas, another might get half of the same pill. Yet another (a third) patient might get a totally different treatment. We do not know without understanding the differences which exist between us.
Are you convinced yet?
What can you do to participate?
Sign up to share your medical history and participate in the well-being of the world by upgrading the medical community's knowledge by shedding light on the exact differences between patient populations (gender, race, ethnicity, etc.). By understanding the differences among us, we arrive more quickly at more precise and effective treatments for each of us.
Related Blog Posts:
NIH Director Updates Congress On Research Progress
Dr. Francis Collins and Bill Gates Discuss Global Health And Genomics
How Much Do New Drugs Cost To Bring To The Pharmacy Counter?
Is Disease Or Treatment Different In Women?
Unraveling The Resistance Of Antibiotics!
How Do Chemists Discover New Drugs? A Brief Introduction!