Where is scientific research at on the issue?
Are advancements in discovery being made to deal with the potential threat?
The short answer is that the discovery process takes time and is complicated. Which is no answer at all. Whereas the long term solution involves research being done. As I explained in a previous post on drug discovery, research advances are arduous and take time. Recently, though, progress has been reported in the scientific community and worth giving a "shout out" about. Below is the short post regarding the advance.
Antibiotic Resistance?
Yes, whenever I hear about antibiotic resistance, I stop and pause for a moment of scare. Then I think about the progress that is being made (hopefully). I cannot have myself worry too much about the issue since I do not perform research directly toward a solution. Although, I can support students who are biochemistry undergraduates and graduate students while educating them on the need and importance of such research. Couple that with a proper training on the scientific instrument needed to perform the research and my job ends there.
Sounds scary right?
Well, not all is held in limbo with regard to antibiotic resistance.
First, what is antibiotic resistance? In order to understand the issue, what is the problem?
Here is an excerpt taken from the 'Wikipedia' page for "Antibiotic Resistance" is shown below:
Antimicrobial resistance (AMR) is the ability of a microbe to resist the effects of medication previously used to treat them.[2][3][4] This broader term also covers antibiotic resistance, which applies to bacteria and antibiotics.[3] Resistance arises through one of three ways: natural resistance in certain types of bacteria; genetic mutation; or by one species acquiring resistance from another.[5] Resistance can appear spontaneously because of random mutations; or more commonly following gradual buildup over time, and because of misuse of antibiotics or antimicrobials.[6] Resistant microbes are increasingly difficult to treat, requiring alternative medications or higher doses—which may be more costly or more toxic. Microbes resistant to multiple antimicrobials are called multidrug resistant (MDR); or sometimes superbugs.[7] Antimicrobial resistance is on the rise with millions of deaths every year.[8] A few infections are now completely untreatable because of resistance. All classes of microbes develop resistance (fungi, antifungal resistance; viruses, antiviral resistance; protozoa, antiprotozoal resistance; bacteria, antibiotic resistance).Antibiotics should only be used when needed as prescribed by health professionals.[9] The prescriber should closely adhere to the five rights of drug administration: the right patient, the right drug, the right dose, the right route, and the right time.[10] Narrow-spectrum antibiotics are preferred over broad-spectrum antibiotics when possible, as effectively and accurately targeting specific organisms is less likely to cause resistance.[11] Cultures should be taken before treatment when indicated and treatment potentially changed based on the susceptibility report.[12][13] For people who take these medications at home, education about proper use is essential. Health care providers can minimize spread of resistant infections by use of proper sanitation: including handwashing and disinfecting between patients; and should encourage the same of the patient, visitors, and family members.[12]Rising drug resistance can be attributed to three causes use of antibiotics: in the human population; in the animal population; and spread of resistant strains between human or non-human sources.[6] Antibiotics increase selective pressure in bacterial populations, causing vulnerable bacteria to die—this increases the percentage of resistant bacteria which continue growing. With resistance to antibiotics becoming more common there is greater need for alternative treatments. Calls for new antibiotic therapies have been issued, but new drug-development is becoming rarer.[14] There are multiple national and international monitoring programs for drug-resistant threats. Examples of drug-resistant bacteria included in this program are: methicillin-resistant Staphylococcus aureus (MRSA), vancomycin-resistant S. aureus (VRSA), extended spectrum beta-lactamase (ESBL), vancomycin-resistant Enterococcus (VRE), multidrug-resistant A. baumannii (MRAB).[15]A World Health Organization (WHO) report released April 2014 stated, "this serious threat is no longer a prediction for the future, it is happening right now in every region of the world and has the potential to affect anyone, of any age, in any country. Antibiotic resistance—when bacteria change so antibiotics no longer work in people who need them to treat infections—is now a major threat to public health."[16] Increasing public calls for global collective action to address the threat include proposals for international treaties on antimicrobial resistance.[17] Worldwide antibiotic resistance is not fully mapped, but poorer countries with weak healthcare systems are more affected.[9] According to the Centers for Disease Control and Prevention: "Each year in the United States, at least 2 million people become infected with bacteria that are resistant to antibiotics and at least 23,000 people die each year as a direct result of these infections." [18]
Is that a comprehensive definition?
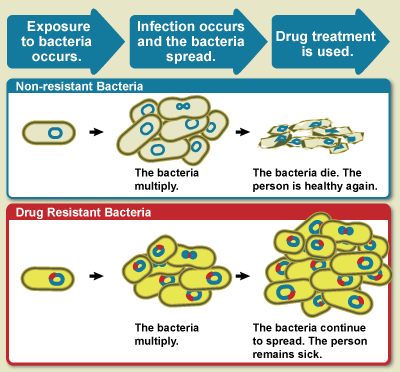
Instead, why not start with a simple pictorial representation of what antimicrobial resistance is. In a previous post on the need for greater science communication, Dr. Tyler Dewitt gave a great explanation of the modes of how virus's and bacteria invade a host organism like the human body. I suggest taking a look at the post. Once the invader (virus or bacteria) enters the system, the invader takes hold (control) of your system. The control allows the invader to make several copies of itself to proliferate and grow to become a problem (onset of disease).
What can be done about such a state?
One methodology that has become increasingly common if the invader is a microbe or bacteria is to administer antibiotics which wipe out the infection. Shown below is a picture taken from the 'Wikipedia' page again for clarity to illustrate the point of action of antibiotics:
Source:By NIAID – NIH
The image above is very simple to understand the action of an antibiotic.
Advances In Antibiotic Resistance
Recently, there has been advances in research surrounding antibiotic resistance that may speed up the ability to deal with the looming threat. In an article from the website 'Sciencedaily.com' titled "Mystery of bacteria's antibiotic resistance unravelled" new developments have been made in unraveling the mode of disabling the effect of antibiotics by researchers. Here is an excerpt discussing the advancement:
One of the mechanisms leading to rifampicin's resistance is the action of the enzyme Rifampicin monooxygenase.Pablo Sobrado, a professor of biochemistry in the College of Agriculture and Life Sciences, and his team used a special technique called X-ray crystallography to describe the structure of this enzyme. They also reported the biochemical studies that allow them to determine the mechanisms by which the enzyme deactivates this important antibiotic.The results were published in the Journal of Biological Chemistry and PLOS One, respectively."In collaboration with Professor Jack Tanner at the University of Missouri and his postdoc, Dr. Li-Kai Liu, we have solved the structure of the enzyme bound to the antibiotic," said Sobrado, who is affiliated with the Fralin Life Science Institute and the Virginia Tech Center for Drug Discovery. "The work by Heba, a visiting graduate student from Egypt, has provided detailed information about the mechanism of action and about the family of enzymes that this enzyme belongs to. This is all-important for drug design."
Before I make a few comments on the success of the discovery in the pipeline to a marketable drug or treatment, I would like to add another excerpt from the same article highlighting the importance of the antibiotic rifampicin is to an array of diseases:
Rifampicin, also known as Rifampin, has been used to treat bacterial infections for more than 40 years. It works by preventing the bacteria from making RNA, a step necessary for growth.The enzyme, Rifampicin monooxygenase, is a flavoenzyme -- a family of enzymes that catalyze chemical reactions that are essential for microbial survival. These latest findings represent the first detailed biochemical characterization of a flavoenzyme involved in antibiotic resistance, according to the authors.Tuberculosis, leprosy, and Legionnaire's disease are infections caused by different species of bacteria. While treatable, the diseases pose a threat to children, the elderly, people in developing countries without access to adequate health care, and people with compromised immune systems.
As you can see, the ability of the antibiotic rifampicin to knock out an array of important diseases cannot be overstated. Therefore, any advancement in understanding modes of action or in this case 'inaction' are critical to drug designers for the future. At this point, you might be wondering what the structure of rifampicin looks like? Shown below is the chemical structure of rifampicin take from 'Wikipedia':
Rifampicin
Source of image: By Vaccinationist - Rifampicin on PubChem
With the discovery of the mechanism by which Rifampicin Monooxygenase deactivates rifampicin's ability to act as an antibiotic, should we all throw our hands up and celebrate?
The discovery of the deactivation mechanism of rifampicin is a major step for drug makers in producing new lines of antibiotics in the future. As I mentioned in a previous blog on drug discovery, the flow of patentable drug includes discoveries made at the university level. This discovery certainly qualifies as one -- certainly. Although, more studies will have to be followed up in order to realize the discovery into a better antibiotic in the future.
I should mention one major point of contention about the discovery of the mechanism. The spectroscopic technique that was used was x-ray crystallography. X-ray crystallography as a technique just celebrated it't 100th year since the discovery of the technique. Here is an excerpt from the 'Wikipedia' page describing the technique of 'x-ray crystallography':
X-ray crystallography is a tool used for identifying the atomic and molecular structure of a crystal, in which the crystalline atoms cause a beam of incident X-rays to diffract into many specific directions. By measuring the angles and intensities of these diffracted beams, a crystallographer can produce a three-dimensional picture of the density of electrons within the crystal. From this electron density, the mean positions of the atoms in the crystal can be determined, as well as their chemical bonds, their disorder and various other information.Since many materials can form crystals—such as salts, metals, minerals, semiconductors, as well as various inorganic, organic and biological molecules—X-ray crystallography has been fundamental in the development of many scientific fields. In its first decades of use, this method determined the size of atoms, the lengths and types of chemical bonds, and the atomic-scale differences among various materials, especially minerals and alloys. The method also revealed the structure and function of many biological molecules, including vitamins, drugs, proteins and nucleic acids such as DNA. X-ray crystallography is still the chief method for characterizing the atomic structure of new materials and in discerning materials that appear similar by other experiments. X-ray crystal structures can also account for unusual electronic or elastic properties of a material, shed light on chemical interactions and processes, or serve as the basis for designing pharmaceuticals against diseases.
The spectroscopic technique is very powerful and is commonly used in a wide range of areas of research for structural determination. One drawback is the constraint of having to grow a crystal -- a rather large crystal to subject the x-rays to in order to obtain a diffraction pattern.
Why does this matter?
One commonly held belief among spectroscopists is that x-ray crystallography is extremely useful in a range of areas as a first step or a confirmation step. The constraint of having to grow a crystal is also a large point of contention regarding the usefulness of the information obtained by the diffraction pattern. Why?
The reason is centered around the fact that processes in the body (i.e., at physiological conditions) are performed in a 'liquid-state' rather than a 'crystalline-state' (i.e., solid state). Scientists argue about the true degree of accuracy of a structure obtained by x-ray crystallography rather than say a structure obtained by nuclear magnetic resonance (NMR) in the liquid state. The structure obtained by NMR is believed to be more representative of the actual conditions (liquid state, pH, temperature, etc.).
Nevertheless, the discovery above is extremely important. The realization of a site of deactivation for rifampicin monooxygenase can now be further explored and compared to other antibiotics. Scientists in industry and academia (university settings) will use incorporate this mechanism into their current understanding and models to produce a better antibiotic.
Further, understanding 'antimicrobial resistance' is a hot topic. Just today, the 'Los Angeles Times' published an editorial discussing two bills that are hitting legislature for consideration. Lets hope the combined efforts of all of these actions, leads to fewer cases of deaths in hospitals along with safer and better antibiotics.
Conclusion...
Should we be celebrating?
The advancement discussed above is cause to celebrate momentarily. Although, as I mentioned in the previous post regarding the drug discovery process, the path is long and arduous. Advancements such as these improve the ability of researchers to add another piece to the puzzle. Given more information, further advances can be pushed even further. Of course, that goes without being said (i.e., thank you captain obvious). Research is a long process and needs a lot of funding and time to test and retest procedures to make sure that scientists get the process right -- to eliminate the problem the first time around. Adjustments often have to be made due to inefficiencies of a given treatment or terrible side effects. Although, with a better understanding of the mode of action and inaction, drug manufacturers create more accurate drugs and research is one step further in understanding how nature operates to take control over our immune systems or subject us to terrible diseases.
Last but not least, the overall importance of writing a post like this is to convey the excitement and importance of such research. To demystify the meaning of "antibiotic resistance" or "antimicrobial resistance." Raising awareness of the magnitude of the issue will hopefully rally support on part of the public (your support) to elevate the need for funding and research into such issues. Do your part. Advocate for science and educate yourself on the successes and challenges (failures, obstacles). Give us some feedback.
Until next time, have a wonderful day!