What Is Milk Composed Of?
What is milk made up of? Hormones, proteins, other large macromolecules that come from an animal? Can we make milk? Yes, humans do -- just look toward any pregnant mother who is nursing a newborn by breast feeding. The definition of "milk" taken from "Wikipedia" is the following:
Milk is a pale liquid produced by the mammary glands of mammals. It is the primary source of nutrition for infant mammals before they are able to digest other types of food. Early-lactation milk contains colostrum, which carries the mother's antibodies to its young and can reduce the risk of many diseases. It contains many other nutrients[1] including protein and lactose.
After reading the excerpt above, why would anyone want to drink milk after reaching adulthood? Milk contains a broad spectrum of macromolecules that are nutritious. What is a "macromolecule"? Here is a definition from "Wikipedia" of a "macromolecule":
A macromolecule is a very large molecule, such as protein, commonly created by polymerization of smaller subunits (monomers). They are typically composed of thousands of atoms or more. The most common macromolecules in biochemistry are biopolymers (nucleic acids, proteins, carbohydrates and polyphenols) and large non-polymeric molecules (such as lipids and macrocycles).[1] Synthetic macromolecules include common plastics and synthetic fibres as well as experimental materials such as carbon nanotubes.[2][3]
The concept of a macromolecule is generalized by the scientific community. Basically, when a molecule is considered a macromolecule if the weight is large (on the order of kilo-Daltons) or made up of subunits (and is still large). What does the concept have to do with milk?
Milk is made up of a distribution of molecules that range in size and shape. Most of the molecules are on the heavier side (proteins) but still milk contains sugars too. And as you will see shortly, the sugars play a large role in supplying the microbes contained in your gut -- which additionally plays a role in building the immune system.
What? Wait.
How did we go from macromolecules to building the immune system?
Fair enough, lets take a couple of steps back in order to move forward toward building the immune system. Starting from the small scale working our way up, the first stop is the definition of a "monosaacharide" shown below -- taken from the "Wikipedia" page:
Monosaccharides (from Greek monos: single, sacchar: sugar), also called simple sugars, are the most basic units of carbohydrates. They are fundamental units of carbohydrates and cannot be further hydrolised to simpler compounds. The general formula is CnH2nOn. They are the simplest form of sugar and are usually colorless, water-soluble, and crystalline solids. Some monosaccharides have a sweet taste. Examples of monosaccharides include glucose (dextrose), fructose (levulose) and galactose. Monosaccharides are the building blocks of disaccharides (such as sucrose and lactose) and polysaccharides (such as cellulose and starch). Further, each carbon atom that supports a hydroxyl group (so, all of the carbons except for the primary and terminal carbon) is chiral, giving rise to a number of isomeric forms, all with the same chemical formula. For instance, galactose and glucose are both aldohexoses, but have different physical structures and chemical properties.
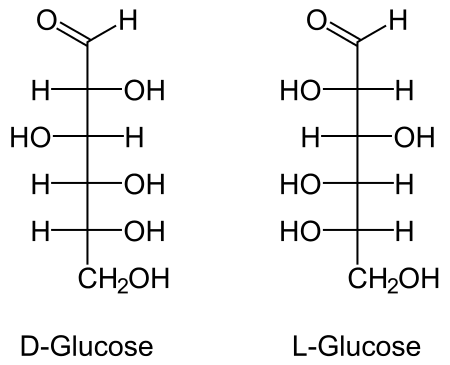
There is a lot of discussion in the media around carbohydrates and fats in regard to nutrition. Now, you can educate other people that carbohydrates are made up of smaller molecular structures like the commonly stated molecule - 'glucose' - whose molecular structure is shown below:
Source: NEUROtiker
From the molecular structure above, the two monosaacharides shown -- D-Glucose and L-Glucose -- are made up of a certain number of carbon atoms, hydrogen atoms, and oxygen atoms. These atoms are indicated by the letters "C", "H", and "O" respectively.
Why did I show you these structures?
The reason is to drive home the point of the variability in nature. While a person who has no training in chemistry might look at the structures above and think there are so many different forms -- how do I memorize or recognize them -- a person with training will see patterns. The first and most important pattern is that all the molecules of life contain carbon, hydrogen, and oxygen at the very least. That is why the study of such molecules is classified in the field of chemistry as "organic chemistry."
What do these molecules have to do with proteins, hormones, other macromolecules?
As shown in the definition above, a macromolecule is made up of smaller molecules. For the molecules associated with life, typically, the basic unit is glucose or another six membered ring of carbon, hydrogen, and oxygen atoms. If you go onto study chemistry, you will find that the arrangements of the atoms on the rings in space give rise to different structures too.
When basic units are bonded together, the result is larger units -- polysaacharides. Monosaacharides are the basic units to build larger structures that are commonly discussed in the news. What?
Yes, as shown in the excerpt above for the definition of a monosaacharide, larger molecular structures such as sucrose and lactose (disaacharides) originate from basic units. Furthermore, large molecular structures such as cellulose and starch are termed polysaacharides. I am sure that the last two polysaacharides have crossed your radar before. Polysaacharides are extremely important in the biochemistry of life. Here is a definition of a "polysaacharide" from "Wikipedia" shown below:
Polysaccharides are polymeric carbohydrate molecules composed of long chains of monosaccharide units bound together by glycosidic linkages and on hydrolysis give the constituent monosaccharides or oligosaccharides. They range in structure from linear to highly branched. Examples include storage polysaccharides such as starch and glycogen, and structural polysaccharides such as cellulose and chitin.
Polysaacharides can be made up of a number of monosaacharides that have been bonded together in specific manner. When the number of simple sugars that are linked together lie in a range from three-ten, the class of polysaacharides is known as "oligosaacharides."
Why am I showing all of these structures which seemingly reduce to carbon, hydrogen, and oxygen atoms?
Alright, I will start to get to the point of the blog post -- the newly discovered benefits of breast milk that have been discovered recently. How? Part of how has been by focusing on the microbiome. What? Turns out that during the analysis of milk, scientists have found a connection between components (oligosaacharides) in milk that work with the microbiome to boost the immune system. WOW. Let me explain with the article which spurred the thought to write this post below.
Breast Milk Boosts Immune System Through The Microbiome?
In an article from "The New Yorker" magazine titled "BREAST-FEEDING THE MICROBIOME" the author Ed Yong provided an excerpt from his new book titled "I Contain Multitudes: The Microbes Within Us and a Grander View of Life." His book, as described by a couple of book review descriptions is a view of the world through the lens of the microbial community. Which is to say, that Ed shows the value that the microbial kingdom brings to the world around us. The book has a release date of August 9th, I have pre-ordered my copy.
Specifically, in the excerpt provided in the article on "The New Yorker" magazine, the author discusses the role of microbes play in regards to breast feeding a baby.
Who would have thought that microbes play any role in the process?
Breast feeding involves the baby and the mother along with the passage of nutrients between the two, right?
According to the author who has interviewed an array of scientists -- whose research is centered around the topic, that view is only part of the picture. In the article, the excerpt chosen from his new book introduces the reader to research on milk that is being conducted at the Foods for Health Institute at University of California at Davis. Milk has a reputation for being a rather simple solution -- which is anything but the case. Here is an excerpt from the article:
Milk is a mammalian innovation, common to platypuses and pangolins, humans and hippos, its ingredients varying according to what each species needs. Human milk is a particular marvel. Every mammal mother produces complex sugars called oligosaccharides, but human mothers, for some reason, churn out an exceptional variety: so far, scientists have identified more than two hundred human milk oligosaccharides, or H.M.O.s. They are the third-most plentiful ingredient in human milk, after lactose and fats, and their structure ought to make them a rich source of energy for growing babies—but babies cannot digest them. When German first learned this, he was gobsmacked. Why would a mother expend so much energy manufacturing these complicated chemicals if they were apparently useless to her child? Why hasn’t natural selection put its foot down on such a wasteful practice? Here’s a clue: H.M.O.s pass through the stomach and the small intestine unharmed, landing in the large intestine, where most of our bacteria live. What if they aren’t food for babies at all? What if they are food for microbes?
This idea dates back to the early twentieth century, when two very different groups of scientists made discoveries that, unbeknownst to them, were closely connected. In one camp, pediatricians found that microbes called Bifidobacteria (“Bifs,” to their friends) were more common in the stools of breast-fed infants than bottle-fed ones. They argued that human milk must contain some substance that nourished the bacteria—something that later scientists called the bifidus factor. Meanwhile, chemists had discovered that human milk contains carbohydrates that cow milk does not, and were gradually whittling this enigmatic mixture down to its individual components, including several oligosaccharides. The parallel tracks met in 1954, thanks to a partnership between Richard Kuhn (chemist, Austrian, Nobel laureate) and Paul Gyorgy (pediatrician, Hungarian-born American, breast-milk advocate). Together they confirmed that the mysterious bifidus factor and the milk oligosaccharides were one and the same—and that they nourished gut microbes.
Obviously, the assumption that milk is a simple solution is disputed by the evidence (scientific research findings) over the years. Although, the benefit of certain ingredients have been an active question over the years -- which research has recently shed light on to settle disputes that have gone on for decades. This is highlighted in the excerpt above with regard to the role of human milk oligosaacharides(HMO's).
The mysterious player in the equation seem to be the bacteria that live in the gut. There is a variety that make up a complete microbiome which help defend the body against foreign invaders. Previous research did not include any consideration of the microbiome playing a role in boosting the immune system. Lately, with the emerging understanding of various microbiota in foods, products, places -- this view is starting to change. More will be said about this in future blog posts.
Earlier in the post, oligosaacharides were introduced as a certain class of polysaacharides. According to the excerpt above, a human mother produces nearly 200 of these oligosaacharides in their breast milk. The reason for the production of these HMO's has been in question since as discovered by the Germans -- babies do not digest these macromolecules.
What was the purpose of producing the oligosaacharides in breast milk if they did not serve as nutrients for the bifidobacteria?
Scientists learned that the HMO's did not serve as nutrients for the bifidobacteria by extracting the the HMO's and feeding them to various bacteria cultures. The results suggested that a "subspecies" of bifidobacteria were fed by the HMO's:
The problem soon became clear: H.M.O.s are not an all-purpose food for Bifs. In 2006, the team found that the sugars selectively nourish one subspecies, Bifidobacterium longum infantis. As long as you provide B. infantis with H.M.O.s, it will outcompete any other gut bacterium. A closely related subspecies, B. longum longum, grows weakly on the same sugars, and the ironically named B. lactis, a common fixture of probiotic yogurts, doesn’t grow at all. Another probiotic mainstay, B. bifidum, does slightly better, but is a fussy, messy eater. It breaks down a few H.M.O.s and takes in the pieces it likes. By contrast, B. infantis devours every last crumb using a cluster of thirty genes—a comprehensive cutlery set for eating H.M.O.s. No other Bif has this genetic cluster; it is unique to B. infantis. Human milk has evolved to nourish the microbe, and it, in turn, has evolved into a consummate H.M.O.vore. Unsurprisingly, it is often the dominant microbe in the guts of breast-fed infants.
B. infantis earns its keep. As it digests H.M.O.s, it releases short-chain fatty acids, which feed an infant’s gut cells. Through direct contact, B. infantis also encourages gut cells to make adhesive proteins that seal the gaps between them, keeping microbes out of the bloodstream, and anti-inflammatory molecules that calibrate the immune system. These changes only happen when B. infantis feeds on H.M.O.s; if it gets lactose instead, it survives but doesn’t engage in any repartee with the baby’s cells. In other words, the microbe’s full beneficial potential is unlocked only when it feeds on breast milk. Likewise, for a child to reap the full benefits that milk can provide, she must have B. infantis in her gut. For that reason, David Mills, a microbiologist who works with German, actually sees B. infantis as part of milk, albeit a part that is not made in the breast.
Wow! The bacteria B. infantis actually communicate with the cells of the human gut to produce a protein to protect the gaps in between them. Thereby protecting access to the bloodstream and protecting against foreign invaders from attacking our immune system. The research behind these results is quite amazing.
This is a complete change in thinking about the role of microbiology in our bodies. Instead of thinking of bacteria that colonizes our gut as "foreign invaders" which our system has to ward off, the colonization is present to promote the health of our immune system by communicating to the gut cells to produce protective proteins. Additionally, this allows the immune system to build itself up by not having to work as hard in the early stages of development. Amazing!
As you can see there are many competing factors that contribute to the health of the microbiome. Aside from feeding the gut bacteria, competition among various cultures of bacteria exists which can contribute to the health of our bodies or have an adverse effect depending on the concentration of the species present.
The idea of stabilizing microbiomes is emerging as an important aspect to our everyday health. More and more data is starting to emerge with the completion of every study. The specificity of the results are astounding too. As an example, here is an excerpt discussing the discovery of the multitude of purposes that the HMO's can serve aside from feeding the B. infantis:
An alternative idea involves diseases. In a group setting, pathogens can easily bounce from one host to another, so animals need better ways of protecting themselves. H.M.O.s provide one such defense. When a pathogen infects our guts, it almost always begins by latching onto glycans—sugar molecules—on the surfaces of our intestinal cells. But H.M.O.s bear a striking resemblance to these glycans, so pathogens sometimes stick to them instead. They act as decoys, drawing fire away from a baby’s own cells. They can block a roll call of gut villains, including Salmonella; Listeria; Vibrio cholerae, the culprit behind cholera; Campylobacter jejuni, the most common cause of bacterial diarrhea; Entamoeba histolytica, a voracious amoeba that causes dysentery and kills a hundred thousand people every year; and many virulent strains of E. coli. H.M.O.s may even be able to obstruct H.I.V., which might explain why more than half of infants who suckle from infected mothers don’t get infected, despite drinking virus-loaded milk for months. Every time scientists have pitted a pathogen against cultured cells in the presence of H.M.O.s, the cells have come out smiling.
Not only do the human milk oligosaacharides not get digested by the babies, the macromolecules serve to feed the existing dominant strain of bacteria (microbiome) B. infantis. Additionally, the macromolecules serve as "decoys" for other dangerous bacteria and viruses (to feed on) to avoid getting absorbed in the gut and intestines. Dangerous bacteria latch onto the oligosaacharides (HMO's) and get swept through our bowels to avoid getting absorbed. The idea behind the route being that if the bacteria or viruses -- foreign invaders -- do not get absorbed into our bodies, the immune system does not need to fight them off. Again, allowing the immune system to build itself up during the developmental stage. Science is amazing.
At the time that the author Ed Yong was writing the book, Prof. David Mills was under the impression (based on studies) that the breast milk from humans only had these nutrients to help B. infantis grow.
According to the "Foods for Health Institute" website, a new article (press release) titled "Cow’s Milk Found to Contain Beneficial Prebiotics for the Infant Microbiome" contains new data that links the same benefits to cows milk too!!! Here is an excerpt describing the evolution of the studies:
In earlier research, these investigators, led by David A. Mills, PhD, had shown that glycoproteins from milk, which contain both protein, and molecules containing multiple sugars, called oligosaccharides, were the source of that nourishment. They also had found that the infant-associated subspecies of the bacterium,Bifidobacterium longum subsp. infantis (B. infantis), produced an enzyme that could cleave the oligosaccharides from the milk glycoproteins, and they had identified that enzyme.For the current study, Mills, who is Professor and Shields Endowed Chair in Dairy Food Science, and his collaborators posited that these oligosaccharides were the food source for B. infantis. They then showed that the enzyme could break down glycoproteins not only from mother’s milk, but from cow’s milk, releasing the oligosaccharides.
The excerpt above goes into slightly more detail than Ed Yong's description regarding the role of the subspecies B. infantis. According the results of the study, the B. infantis produces an enzyme that can cleave (or cut like scissors) the macromolecules into pieces (oligosaacharides are one such piece) which can be used as a "decoy" for dangerous bacteria to latch onto. The oligosaacharides can also serve as nutrients for B. infantis.
I wonder how much time went and money went into this research. The amount of testing (growing bacterial cultures, staining, etc.) and characterization (genetic profiling) must have been extremely time consuming.
Conclusion...
The complexity of milk shall never be questioned again after reading this blog post. Furthermore, the role of the microbiome on human health is just starting to be realized. Emerging research data is pointing toward the microbiome playing a vital role in our health. This is a complete reversal to the prevailing view (of being foreign invaders) that occupied scientists minds in the previous decades. We have just hit the 'tip of the iceberg' so to speak in terms of research and the prospects of the numerous benefits of the microbiome.
As highlighted in the article, there is a large amount of complexity in the process of keeping our immune system healthy. In the work above, the unveiling of cleaving glycan molecules precisely in order to provide nutrients for the bacteria that are colonizing the gut show the amazing reach and range of research in tackling complex questions. Questions like the role of various bacteria in our system and the communication between them. The discussion above highlights the need for scientists to keep forging forward to push the boundaries of our knowledge. As the boundary gets pushed, the results have an impact through new technologies and products to improve our health.
Who would have thought that the microbiome talked to our gut cells?
The gut cells make proteins as a result of such communications?
Stop and think about this for a moment. These results suggest that there an enormous amount of forms of communication between species that have been overlooked or remain to be studied.
What other communication have we missed so far in our research?
With regard to the human body and the ability to supply the proper nutrients, I am astonished. Scientists continue to amaze me with the results and their continuous motivation to pursue the answers that are hidden within the body. I am left speechless after writing the blog post. Questions remain in my mind:
How does the body learn to adapt to the microbiome present in the stomach?
How is that adaptation communicated to the brain?
There are so many unanswered questions that remain to be answered by science. Until next time, have a great day!!
No comments:
Post a Comment