Nanotechnology ("nanotech") is manipulation of matter on an atomic, molecular, and supramolecular scale. The earliest, widespread description of nanotechnology[1][2] referred to the particular technological goal of precisely manipulating atoms and molecules for fabrication of macroscale products, also now referred to as molecular nanotechnology. A more generalized description of nanotechnology was subsequently established by the National Nanotechnology Initiative, which defines nanotechnology as the manipulation of matter with at least one dimension sized from 1 to 100 nanometers. This definition reflects the fact that quantum mechanical effects are important at this quantum-realm scale, and so the definition shifted from a particular technological goal to a research category inclusive of all types of research and technologies that deal with the special properties of matter which occur below the given size threshold. It is therefore common to see the plural form "nanotechnologies" as well as "nanoscale technologies" to refer to the broad range of research and applications whose common trait is size. Because of the variety of potential applications (including industrial and military), governments have invested billions of dollars in nanotechnology research. Until 2012, through its National Nanotechnology Initiative, the USA has invested 3.7 billion dollars, the European Union has invested 1.2 billion and Japan 750 million dollars.[3]Nanotechnology as defined by size is naturally very broad, including fields of science as diverse as surface science, organic chemistry, molecular biology, semiconductor physics, microfabrication, molecular engineering, etc.[4] The associated research and applications are equally diverse, ranging from extensions of conventional device physics to completely new approaches based upon molecular self-assembly, from developing new materials with dimensions on the nanoscale to direct control of matter on the atomic scale.
Do you believe me now?
In the following paragraphs, I want to introduce you to recent research that is shedding light on the mysteries of building solid structures one atom at a time.
Building Nanoparticles!
In a recent article from the online journal "Lab Manager" titled "Scientists Determine Precise 3-D Location and Identity of All 23,000 Atoms in a Nanoparticle" research was reported on regarding figuring out the precise position of 23,000 atoms in a nanoparticle. Here is an excerpt introducing the research in the article:
Scientists used one of the world’s most powerful electron microscopes to map the precise location and chemical type of 23,000 atoms in an extremely small particle made of iron and platinum.The 3-D reconstruction reveals the arrangement of atoms in unprecedented detail, enabling the scientists to measure chemical order and disorder in individual grains, which sheds light on the material’s properties at the single-atom level. Insights gained from the particle’s structure could lead to new ways to improve its magnetic performance for use in high-density, next-generation hard drives.What’s more, the technique used to create the reconstruction, atomic electron tomography (which is like an incredibly high-resolution CT scan), lays the foundation for precisely mapping the atomic composition of other useful nanoparticles. This could reveal how to optimize the particles for more efficient catalysts, stronger materials, and disease-detecting fluorescent tags.
One of the many benefits of the emerging field of nanotechnology is to understand matter at an extremely small scale. The nano scale, as mentioned above, has one dimension on the order of a billionth of a meter -- that is 1/1,000,000,000 meter...which is small. Instructors typically teach chemistry on the macroscale or bulk scale. Right about now, you might be thinking the following:
What do you mean Mike by the "bulk scale" or "macroscale"?
Introduction: "Bulk Phase" or "Bulk Scale" (i.e. "macroscopic scale")
Good question. Chemistry reactions happen on the "macroscopic scale". Another name for the scale is the "bulk phase" or "bulk scale". The unit of quantity used in discussing reactions is called the 'mole'. The 'mole' in chemistry is equivalent to 'a dozen' used in everyday life. A dozen corresponds to the quantity of 12. A mole corresponds to the following number -- Avogadro's number shown below:
602,300,000,000,000,000,000,000 molecules (or atoms) = 1 mole of molecules (or atoms)
This number when used to discuss chemistry is cast in context to the number of atoms or molecules contained within a mole. For example, if we discuss the chemical reaction of the combustion of methane gas, the following chemical reaction could be written as:
Source: Wikipedia
In the reaction above, two different representations exist. The top is a molecular representation of the reaction. Whereas, on the bottom, the written reaction is represented. Notice that in front of "02" there is a number "2". That number signifies the number of 'moles' -- which I will touch on shortly. A chemist would read the above reaction as follows:
1 mole of methane gas reacts with 2 moles of oxygen gas to form 1 mole of carbon dioxide and 2 moles of water!
When discussing chemical reactions like the combustion of methane gas listed above, typically, the amounts of combustible material and the masses liberated are expressed in units of 'moles'. A 'mole' is a very large number as shown above 6.023 with 23 zero's after the decimal place. This gives us an idea of just how small atoms are. To give you an idea of viewing a chemical reaction using molar amounts (moles), view the following combustion reaction shown below:
In the video above, the reaction of the combustion of methane gas trapped in a bubble of soap. The amount of methane molecules contained in the bubble are on the order of Avogadro's number (some percentage of a mole). Viewing chemistry reactions from this "macromolecular" frame is remanent of the historical founding of chemistry.
For the past few centuries, chemistry has been taught and performed on a large scale - molar scale. This is referred to as "bulk phase" chemistry. Alternatively, on the other side of the scale -- down in scale -- lies the world of 'nanotechnology'. The bridge between the 'nanoscale' and 'bulk phase' is still being defined.
Bulk Phase vs. Nanoscale
As I mentioned above, the reactions are written in what seems to be the simplest form. Yet, the quantities of reactants (reacting molecules) and products (molecules produced) are expressed in terms of 'moles' -- which is a large number.
What is fascinating is that the best computational power emerging presently is no where near able to represent a typical chemical reaction with molar quantities. That would take an unheard of amount of computing power to perform. When computational chemist simulate reactions, the number of molecules are usually small as described in the introduction of the "Wikipedia" page for "computational chemistry":
Computational chemistry is a branch of chemistry that uses computer simulation to assist in solving chemical problems. It uses methods of theoretical chemistry, incorporated into efficient computer programs, to calculate the structures and properties of molecules and solids. It is necessary because, apart from relatively recent results concerning the hydrogen molecular ion (dihydrogen cation, see references therein for more details), the quantum many-body problem cannot be solved analytically, much less in closed form. While computational results normally complement the information obtained by chemical experiments, it can in some cases predict hitherto unobserved chemical phenomena. It is widely used in the design of new drugs and materials.Examples of such properties are structure (i.e., the expected positions of the constituent atoms), absolute and relative (interaction) energies, electronic charge density distributions, dipoles and higher multipole moments, vibrational frequencies, reactivity, or other spectroscopic quantities, and cross sections for collision with other particles.The methods used cover both static and dynamic situations. In all cases, the computer time and other resources (such as memory and disk space) increase rapidly with the size of the system being studied. That system can be one molecule, a group of molecules, or a solid. Computational chemistry methods range from very approximate to highly accurate; the latter are usually feasible for small systems only. Ab initio methods are based entirely on quantum mechanics and basic physical constants. Other methods are called empirical or semi-empirical because they use additional empirical parameters.Both ab initio and semi-empirical approaches involve approximations. These range from simplified forms of the first-principles equations that are easier or faster to solve, to approximations limiting the size of the system (for example, periodic boundary conditions), to fundamental approximations to the underlying equations that are required to achieve any solution to them at all. For example, most ab initio calculations make the Born–Oppenheimer approximation, which greatly simplifies the underlying Schrödinger equation by assuming that the nuclei remain in place during the calculation. In principle, ab initio methods eventually converge to the exact solution of the underlying equations as the number of approximations is reduced. In practice, however, it is impossible to eliminate all approximations, and residual error inevitably remains. The goal of computational chemistry is to minimize this residual error while keeping the calculations tractable.
With the emergence of the computer and the ability to handle calculations of properties of molecules and reactions, chemists are now able to look at chemistry (reactions, properties, phases, etc.) from the ground up. That is, from a couple of atoms or molecules reacting toward groups or phases reacting. The complexity and time scales with the number of atoms or molecules. Here is where the dilemma lies.
A typical chemical reaction like the combustion of methane gas trapped in the bubbles of soap (shown above) occurs on the 'macroscopic scale' whereas the ability of a computational chemist still resides on the molecular scale. Ideally, chemists would enjoy the possibility of simulating the macroscopic scale in terms of reactions, phases, properties of systems, etc.
Currently, researchers are trying to achieve defining the boundary between the 'nanoscale' and 'bulk phase'. Researchers from the University of Hyderabad in India have been studying the boundary between the two in an article titled "An experimental criterion for the nano-to-bulk phase transition":
The continued interest in nanostructured, nanocrystalline and nanograined materials is not surprising due to the very important technological implications of these materials [1]; [2]; [3]; [4]; [5]; [6] ; [7]. It is also true that the effect of size on physical behaviour of materials has thrown up several fundamental questions that point to the fact that the nanophase has to be treated as a separate state of matter. Although the prediction by Gleiter [8] ; [9] to this effect was made many years ago, there have been very few attempts at arriving at a universal definition of the onset of the nanophase that cuts across physical phenomena. Kreibig and Vollmer [10] have suggested that 10^7 atoms are approximately the upper limit for metal nanoclusters to exist.In our previous paper [11], we have given theoretical as well as experimental evidence for the nano to bulk transition to be regarded as a phase transition. It was demonstrated that the chemical potential of bosons trapped in a harmonic potential shows a discontinuity as a function of the number of particles in the system. It was further shown that bulk-like behaviour is exhibited by the system if the number of particles is of the order of 10^6 or greater. Indeed, this can also be expressed in terms of the ratio of crystallite volume, V, of the experimental sample and the volume of the unit cell, Vc. Significantly, even in this case for values of the ratio >10^6, the materials exhibit bulk-like behaviour. This was demonstrated to be true for a variety of physical phenomena. However, the nature of the phase transition was not discussed in the earlier work. In the current work, the nature of the phase transition is discussed leading to a possible experimental criterion for the onset of nanophase is proposed.
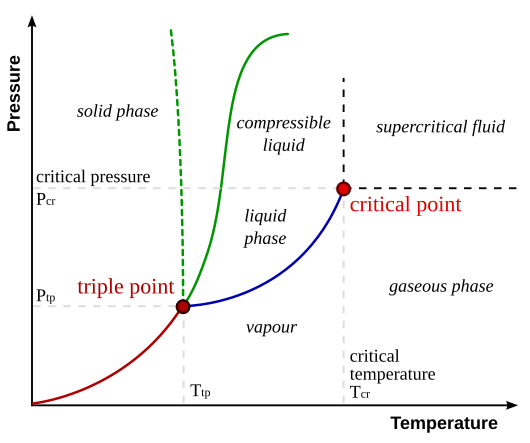
Treating the 'nanoscale' and 'bulk scale' as different phases is an interesting concept. Traditionally, chemists think of different phases as the three most common: liquid phase, gas phase, and solid phase. The three phase diagram for a single pure substance is shown below:
Source: Matthieumarechal
Notice how there are different phases shown in the diagram above. To the left of the curve (high pressure, low temperature) is the 'solid phase'. Moving right (increase in temperature) is the 'liquid phase'. Finally, moving down from the 'liquid phase' (decreasing pressure) is the 'gaseous phase'. Each of the phases have distinct properties of matter.
Moving from the 'bulk phase' to the 'nanoscale' is equivalent to increasing the magnification on matter. Looking at fewer molecules, yet taking into account the distinct properties of individual atoms or molecules. Performing chemistry on the 'nanoscale' requires chemists (and physicists) to consider properties of elements on a small scale. Meaning that when performing the research as highlighted in building up a nanoparticle, scientists learn more about how individual atoms or molecules impact large scale properties.
The nanoscale offers the ability to tweak molecules - atom by atom which is unique compared to previous centuries of science. Additionally, learning how the distinct properties (electronic configuration, spatial configuration, thermodynamic properties) of each atom or molecule offer greater insight into properties that have never before been able to be studied. Previously, the properties were discussed in a statistical fashion as part of a group behavior.
Nanotechnology has pushed that boundary forward and continues to do so.
Conclusion...
Manipulating matter on the 'nanoscale' involves building up matter from the ground up. Which is to say, atom by atom or molecule by molecule. Strange properties are starting to be discovered. As I mentioned earlier, when chemistry is performed between small amounts of molecules or atoms, the chemical properties of the elements involved come into play a greater role. At this scale, there is a large amount of room to expand our knowledge. Groups of atoms or molecules can have a different reaction coordinates from that of the 'bulk phase' -- moles reacting. Why is this? Still open ended. How do individual atoms or molecules interact on the nanoscale which gives rise to 'macroscopic' properties? Still open ended. The field of nanotechnology has a tremendous amount of benefits to offer as chemists learn the effects of various properties on a microscopic scale.
Whereas, chemistry which occurs on a 'macroscopic' scale involves greater quantities of molecules and atoms. Statistics plays a greater role in the description of the behavior of atoms and molecules on the macroscopic scale. Each scale has its place within the context of chemistry. Chemistry which occurs on the microscopic scale might be very different than that which occurs on the macroscopic scale. An example of this might be stirring a cup of coffee. How would the thermodynamics of the stirring change if the scale of the volume were increased to a 1000 liter reaction vessel? Investigating differences remains to be conquered. In the meantime, bridging the gap between the nanoscale and the macroscopic scale involves developments like building up nanoparticles and following their behavior over time (stability, etc.).
Until next time, have a great day!

No comments:
Post a Comment