Is there any technology to prevent fires in Lithium Ion batteries?
Recent research suggests that new research shows promise of preventing future fires. In the paragraphs below, I will explain with a few pictures taken from the original article of interest.
Why Do Batteries Catch Fire?
A diagram of a lithium ion battery is shown below:
Source: HowStuffWorks.com
A diagram of the setup of a lithium battery circuit is shown above. The overarching idea behind a battery is to convert chemical energy into electrical energy used to power a circuit (a load) in an electrical device or through the wall socket. In the diagram above, the generic schematic is shown for any battery using lithium ions in the present case. Here are the steps operation to generate electricity from a battery:
1) On the left hand side at the electrode labeled "Anode" an oxidation reaction occurs which generates "free electrons" to move up through the circuit (or load -- i.e., cellphone, iPad, flashlight, etc.).
2) Once the reaction is complete, the lithium ions (now positively charged after losing an electron -- negatively charged) diffuse back through the middle divider (called the separator) to the other side of the battery.
3) When the lithium ions (positively charged) reach the opposite electrode called the "Cathode" and proceed through a reaction called a reduction reaction -- in which the positive ion is reduced back to a neutrally charged lithium ion.
Note: these reactions are reversible giving rise to the ability to recharge the battery -- restore charged state.
In the diagram above, the electrodes are represented by "poles" but in reality, the design of electrodes consists of layers of 'electrodes'. That will be important in understanding the ingenuity of the research results below in the next section.
Problems with the lithium ion battery are centered around the fact that the battery catches on fire. That is a major problem for a car own, a pilot of a commercial jet, and other industries on which depend on lithium ion batteries. The following question comes to mind:
How do the batteries catch on fire?
What is the major cause of fires?
The major cause of the fires are related to the rate of charging and discharging of the lithium ion battery. According to an article in the magazine the 'Economist' titled "Why lithium batteries keep catching fire" the reason why the lithium ion batteries catch fire are related to the following properties of the material:
The attraction of a lithium-ion battery is that lithium is the least dense metallic element, which means that weight-for-weight it can pack more power than other types of battery. But lithium is also a highly reactive substance; it belongs to the alkali metal group, which contains sodium and potassium, the high reactivity of which will be familiar from school chemistry classes. Like all batteries, lithium ones consist of two electrodes separated by an electrolyte. Typically for a lithium cell the electrolyte is a solution of lithium salts and organic solvents. When the battery is charged, lithium ions are driven from the electrolyte into a carbon anode. When the battery is discharged they flow back, creating a balancing flow of electrons in a circuit that powers the device. The trouble comes about if there is a small fault or damage is caused to the extremely thin separators that keep the elements of the battery apart. This can lead to an internal short-circuit and a subsequent build-up of heat. This can trigger what is known as a “thermal runaway” in which the battery overheats and can burst into flame. That can cause adjacent battery cells to overheat, which is why groups of cells in some battery packs (such as those used in Tesla cars) are kept in separate protective compartments. Lithium batteries can also be damaged by using them in hot environments, and by excessive discharging and charging—which is why most lithium batteries contain special circuits to prevent this. Catching fire if something goes wrong, then, is in their nature.
Does that explanation make sense?
In order to understand the reason for "thermal runaway" the picture of the battery that we need to have is of a "layered" series of electrodes which sandwich a "separator". Think of a stack of credit cards in your wallet. How each of the cards have their own slot (pocket) in your wallet. The middle pocket would be represented by the "separator" in the above diagram. That would make a thin battery right? Well, look inside of your cellphone some time and you will see that the battery is quite thin.
This means that any "point defect" or misalignment of the electrodes could potentially serve as a point to "short circuit" the battery and cause thermal runaway. Any bump or irregularly rough surface that makes up an electrode can serve as a point for thermal runaway. Furthermore, during the process of charging and discharging, "plating" of atoms occur at either end of the battery at the electrodes during their respective reactions (i.e., oxidation and reduction). Therefore, if the lithium ions or atoms do not attach to the electrode in a smooth manner, then the surface will have defects which can serve as a point for thermal runaway.
In the image below, the surface of the lithium electrode can be seen and is labeled on the right-hand side of the image. The bump or "spur" growing off of the surface of the lithium electrode can serve as a point for 'short circuiting' or 'thermal runaway.'
Source: Physics today
The growths or 'spurs' have been a problem for researchers of battery technology for quite a long time. Designing a battery which controls growth and simultaneously charges/discharges quickly is very difficult. Like everything else in the world, eventually designers reach a point where they must trade-off one advantage for a disadvantage. Consumers cannot have a fast charging/discharging battery which is perfect and without defects. Which is why the results of the research below are promising.
Now that the basic operation of the lithium battery is known along with the major source of fires, lets explore the new research below which could potentially mitigate the problems mentioned above.
How To Prevent Fires Inside Batteries?
As I mentioned above, recent research has shown the possibility of preventing fires in batteries. In an article from the website "ChemistryWorld" titled "Fire-fighting lithium–ion battery doesn’t compromise on performance" new research surrounding a "built-in" flame retardant system has been discovered. Here is an excerpt describing how the flame retardant will work below from the article:
Now, a team of researchers from Stanford University, US, has developed a safety mechanism that could counter the flammable nature of the liquid organic electrolytes used in these batteries. The team’s work focuses on the batteries’ separator – a membrane which is used to stop a battery’s electrodes from coming into contact, while still allowing charge-carrying ions to move through the cell. In the new separator, a flame retardant phosphate is enclosed in an electrospun polymer casing. If the battery overheats, the casing melts and releases the phosphate into the liquid electrolyte, preventing or extinguishing any flames.‘Generally, people need to make a trade-off between battery electrochemical performances – capacity, cycle life, etcetera – and flame retardancy of lithium ion batteries, as the flame retardants that are popularly used can significantly damage the batteries,’ explains Yi Cui, who led the research. ‘Using our “smart” separators, the batteries’ electrochemical performances will not be affected… However, once there is potential thermal runaway, the flame retardancy property will be activated, and nip the fire/explosions in the bud.’
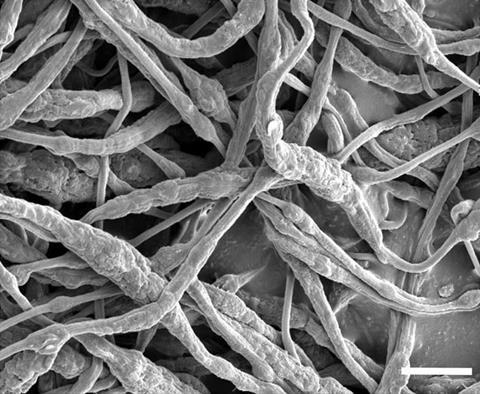
The process by which the material is made is to 'electro-spin' the polymer (the flame retardant) onto the material that acts as a separator. Here is an image of the polymer coating taken from the article but shows a highly magnified picture of the coated fibers below:
The image above shows a rather messy view of the new coated material. Although, the resolution is a fraction of a strand of hair. Yes, you did read correctly. In the lower right-hand corner of the image is a 'white bar' which represents the 'scale' of the image. The original research article lists the scale at 5 micro-meters. A strand of hair is around 70 micro-meters -- that is 0.000070 meters versus 0.000005 meters. Most materials look like the one above at that resolution.
Now that you have an idea of what the material looks like at an up close (microscopic) level, lets look at the system at the macroscopic level. Shown below is video which was embedded in the article mentioned above from "Chemistry World":
Preventing lithium ion batteries from combusting from Chemistry World on Vimeo.
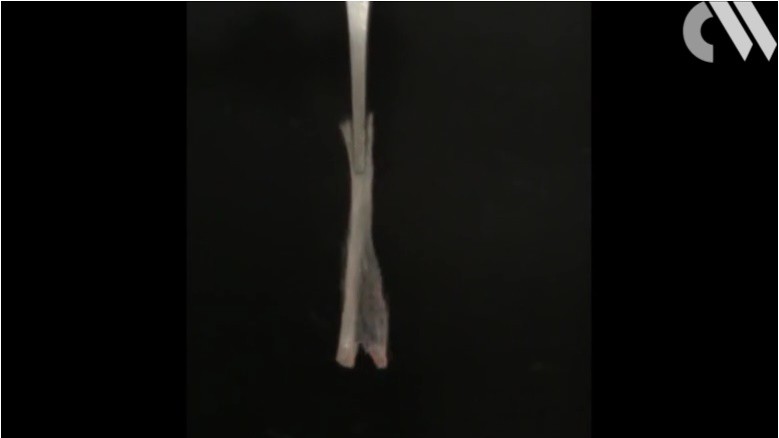
The video above was short. You might miss the effect of the 'flame retardant' (polymer) coating if you were not watching closely. In light of this observation, I decided to break up the video into screenshots below to illustrate the point. Starting with the first image which is of the fiber before combustion:
Upon inspecting the image above, you will notice that the flame is right underneath the fiber -- which does not contain any flame retardant coating. Next, the fiber catches fire and starts to burn up the fiber strands as shown below:
Notice how the flame immediate catches on to the entire bundle of fibers. Now, if the fibers were coated in the polymer which contains flame retardant, the situation would be much different. Below is the same bundle of fibers -- except the coating of flame retardant has been applied to the bundle:
You might be thinking that the flame will catch onto the bundle and ignite. Although, you would be wrong. In the image below, the flame tries to catch on and work up the bundle -- not successfully as shown below:
The flame tries to catch onto any part of the fiber. The flame continues to move up the bundle of fiber with no success of actually igniting due to the release of the flame retardant in the polymer coating. Eventually, the flame is extinguished by the flame retardant that has been released inside of the polymer coating. The coated bundle of fibers remain unharmed as shown below:
These results are promising since the 'separator can play a large role in diffusing a fire that starts in a battery. From the diagram of the lithium battery taken from the website "How Stuff Works" above, you might be asking yourself how can a coated "separator" stop a fire that originates at the electrodes. In the diagram above, there appears to be sufficient space. That is, the battery appears to be a box with space between the electrodes and the separator.
Anyone who owns a cellphone and has taken out the battery will understand that space is very limited. In the image below, the diagram for a lithium ion battery is taken from a cell phone:
Source: Ashland
With the diagram above, the space restriction is very apparent. Therefore, if the separator in the diagram above was coated with a flame retardant inside of a polymer, then any potent fire would be immediately extinguished.
Conclusion...
Lithium ion batteries have been around for decades. They have been overhauled and studied to perfect a battery that is suitable for the cell phone and other devices where space is limited but power is much needed. The problems that scientists are overcoming today were present from the beginning. Although, with the emergence of the field of 'materials science' -- there are new possibilities present that were seemingly unknown to us decades ago.
The possibility of incorporating a flame retardant into a polymer that will be released on contact with heat is extremely promising to a field which has revenue loss in the millions due to this problem. Hopefully, there will be other systems which will work just as well and we can mitigate any lethal problem associated with lithium ion batteries. To date, the lithium ion battery is most promising. Other systems have been proposed, yet are still in the research phase. Stay tuned.
Until next time, Have a great day!







No comments:
Post a Comment